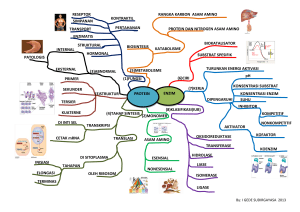
METABOLISME
advertisement

METABOLISME ASAM AMINO KIMIA BIOLOGIS 2011 Inadequate dietary protein is still a major world problem Two-year old child with kwashiorkor, before and two weeks after start of treatment with good protein. Which is before and which is after? KWASHIORKOR - protein deficiency but adequate calories. Described in 1930s as “sickness of older child when new baby is born”, in language of Ga tribe of gold coast (now Ghana). Characteristic edema. 2 Protein malnutrition, continued FAMINE EDEMA Cause: inadequate synthesis of plasma proteins, especially albumin, so that osmotic pressure is not maintained and fluid escapes into tissues. Body water in extracellular space is increased relative to body weight. Extracellular water: Normal ~23.5% Kwashiokor ~30% 3 Protein malnutrition, continued Protein-Energy Malnutrition, Aka Marasmus, Protein-Calorie Deficiency, starvation. Other nutrients (vitamins and minerals) are also likely to be deficient. Starvation is usually the result of war, civil strife, drought, locusts. It especially affects infants and children; growth is slowed, infections and other diseases are common. NY Times, 4/17/00 Ethiopian child 4 Protein malnutrition, continued Such extreme forms of malnutrition are rare in US, but protein deficiency can occur among: • Pregnant and lactating women, unless they increase their protein intake. • Individuals with eating disorders (bulimia, anorexia). • Elderly and chronically ill individuals who have lost interest in eating. • Chronic alcoholics and substance abusers. • Hospital patients with major protein needs and limited capacity for intake (e.g, post-surgery, severe burn victims). • Patients with genetic disorders in amino acid absorption or metabolism. 5 Dietary protein is the source of essential amino acids Dietary proteins provide the amino acids that humans cannot synthesize - the “essential” amino acids. The “non-essential” amino acids can be synthesized endogenously from intermediates of glycolysis or the TCA cycle. Essential Arginine (for children only) Histidine Isoleucine Leucine Lysine Methionine Phenylalanine Threonine Tryptophan Valine Non-essential Alanine Asparagine Aspartate Cysteine Glutamate Glutamine Glycine Proline Serine Tyrosine Mnemonic for essential amino acids: PVT TIM HALL 6 How much protein do we need? • In contrast to fat and glucose, there is no significant storage pool for amino acids; we must consume protein daily. • Requirement for protein depends on age, sex, activity. • Proteins differ in content of essential amino acids as well as digestibility. Diets that rely on a single source of protein may be out of balance with our nutritional needs. ALLOWANCE FOR PROTEIN AGE g/kg g/day Infants (0-1) ~2.2 6.5-20 Children (1-10) 1.8 - 1.25 20- 38 Teens (11-18) 45-55 1.0 - 0.8 Adults (male) 0.8 56 (female) 0.8 44 Pregnant or lactating - 20 - 30% more Athletes REQUIREMENT OF PROTEIN FROM DIFFERENT SOURCES (g/day for 70 kg human) Meat/fish/eggs/milk Non-vegetarian mixed diet Mixed vegetables Single vegetable* ~ 20-25 ~ 25-30 ~ 30-35 up to 75 * Except for soybeans 1.2 -1.7 7 PROTEIN AND AMINO ACID METABOLISM dietary protein Nitrogen balance endogenous proteins digestion amino acids a-ketoacids, NH3 other N compounds glucose, lipids energy urea In N balance excretion = intake (healthy adult) Positive N balance excretion < intake (growth, pregnancy, tissue repair) Negative N balance excretion > intake (malnutrition, starvation illness, surgery, burns) Nitrogen excretion 8 PROTEIN AND AMINO ACID METABOLISM dietary protein endogenous proteins DIGESTION TRANSLATION amino acids a-ketoacids, NH3 other N compounds glucose, lipids energy urea Nitrogen excretion Dietary protein is first hydrolyzed to amino acids, then rebuilt into endogenous protein by translation. 9 Digestion • Mouth: chewing, degradation of starch by amylase make proteins more accessible. • Stomach: acid pH denatures proteins; activates pepsinogen to cleave itself to pepsin, which initiates proteolysis. • Pancreas (exocrine): secretion of trypsinogen, chymotrypsinogen, proelastase, procarboxypeptidase (inactive proenzymes) • Duodenum: peptides from pepsin action stimulate release of cholecystekinin (pancreozymin). Cholecystekinin stimulates release of pancreatic proenzymes and of enteropeptidase, a protease secreted by cells of the duodenum. 10 Digestion • Duodenum: enteropeptidase activates trypsinogen to trypsin. Trypsin activates the other proteases, each of which has different specificity. Dietary proteins converted to peptides and free amino acids. • Small intestine: larger peptides are degraded on the surface of intestinal epithelial cells, which absorb amino acids and small (di- and tri-) peptides. Cytoplasmic peptidases complete conversion of peptides to amino acids, which can enter the circulation. 11 Protein and amino acid metabolism dietary protein endogenous proteins PROTEIN TURNOVER amino acids a-ketoacids, NH3 other N compounds glucose, lipids energy urea Nitrogen excretion 12 Siklus Nitrogen Katabolisme Protein Sumber : diet, degradasi protein dalam tubuh Protein dicerna terlebih dahulu sebelum absorbsi Proses cerna : mulut, lambung, pankreas, dan usus halus Pencerna : asam lambung dan berbagai enzim protease Hasil akhir : asam amino bebas Transport : berbagai cara; memerlukan energi atau tidak memerlukan energi Pencernaan Protein Protein Diet Protein Tubuh Pool Asam Amino Sintesis Protein: Asam amino nonesensial Protein baru (struktural, enzim, hormon) NH3 Asam Keto Senyawa nitrogen lain: Heme, Purin, Pirimidin, dan Kreatin Siklus Krebs Siklus Urea Urea CO2 + H2O + ATP Metabolisme Asam Amino Lokasi: intraselular Tahapan: Pelepasan gugus α-amino (transaminasi & deaminasi oksidatif) Gugus amino digunakan untuk biosintesis asam amino, nukleotida, dll; atau disekresikan dalam bentuk urea (siklus urea) Asam α-keto (rangka karbon) dipecah menjadi senyawa lain: glukosa, CO2, asetil Ko-A, atau badan keton Rangka karbon Amino Asam amino Glukosa Siklus Urea UREA Keton AsetilKoA CO2 Katabolisme Asam Amino Transaminasi: transfer gugus amino ke asam αketoglutarat menghasilkan asam glutamat Deaminasi Oksidatif: Pemecahan Glutamat menjadi amonia dan regenerasi α-ketoglutarat Membutuhkan enzim glutamat dehidrogenase α-ketoglutarat digunakan kembali dalam reaksi transaminasi Siklus Urea Amonia hasil dari pemecahan glutamat digunakan untuk sintesis asam amino baru, sintesis nukleotida, atau senyawa amino lain (porfirin, dll) Amonia berlebih diekskresikan dalam bentuk urea (pada primata) melalui siklus urea Reaksi siklus urea 1 : Karbamoil fosfat sintase 1 kondensasi CO2 dengan amonia → karbamoil fosfat 2 : Ornitin transkarbamoilase kondensasi ornitin dengan karbamoil fosfat → sitrulin 3 : Argininosuksinat sintetase Kondensasi sitrulin dengan aspartat → argininosuksinat 4 : Argininosuksinase Pemecahan argininosuksinat → fumarat dan arginin 5 : Arginase Pemecahan arginin (dengan bantuan H2O)→ urea dan ornitin 4 5 3 2 1 Siklus Urea dan Siklus Krebs berkaitan Katabolisme rangka karbon asam amino Rangka karbon 20 asam amino mengalami metabolisme lanjut yang berbeda Terdiri dari 2 kelompok besar Ketogenik: didegradasi menjadi senyawa antara metabolisme asam lemak; asetil-KoA atau asetoasetat Glukogenik: didegradasi menjadi senyawa antara glikolisis atau SAS; piruvat, α-ketoglutarat, SuksinilCoA, Fumarat, dan oxaloasetat Alanin, Sistein, Glisin, Treonin, Triptofan, Serin Glukosa Asparagin, Aspartat Isoleusin, Leusin, Lisin, Treonin Asetoasetat Leusin, Lisin, Fenilalanin, Triptofan, Tirosin Aspartat, fenilalanin, Tirosin Isoleusin, Metionin, Valin Arginin, Glutamat, Glutamin, Histidin, Prolin AA esensial Degradasi menjadi Arginin Fenilalanin α-ketoglutarat Fumarat, asetoasetil-KoA Histidin Isoleusin Keto Gluko √ √ √ α-ketoglutarat Suksinil-KoA, asetil-KoA √ √ √ Leusin Asetil-KoA, asetoasetil-KoA √ Lisin Asetoasetil-KoA √ Metionin Treonin Suksinil-KoA Suksinil-KoA, piruvat Triptofan Piruvat, asetil-KoA, asetoasetilKoA Suksinil-KoA Valin √ √ √ √ √ AA nonesensial Alanin Asparagin Degradasi menjadi Keto Gluko Piruvat Oksaloasetat √ √ Aspartat Glisin Oksaloasetat, fumarat Piruvat √ √ Glutamat α-ketoglutarat √ Glutamin α-ketoglutarat √ Prolin Serin α-ketoglutarat Piruvat √ √ Sistein Tirosin Piruvat Asetoasetil-KoA, fumarat √ √ √ Biosintesis Asam Amino Fenilalanin • Semua asam amino disintesis dari senyawa antara, kecuali tirosin disintesis dari asam amino esensial fenilalanin • Asam amino esensial: untuk sintesis protein, tidak dapat dibuat sendiri oleh tubuh, terdapat pada makanan • Asam amino non esensial : dapat dibuat oleh tubuh O2 Fenilalanin hidroksilase H2O Tirosin PKU (PhenylKetonUria) : Lack of Phenylalanine hidroxylase *Asam amino esensial Asam amino yang berasal dari 3Fosfogliserat: Serin Sistein Glisin Asam amino yang berasal dari aspartat: Lisin Metionin Treonin Asam amino yang berasal dari piruvat: Leusin Isoleusin Valin Asam amino aromatis: Tirosin Fenilalanin Triptofan Chorismate: Prekursor Asam Amino Aromatis - There is a single precursor for all ‘standard’ aromatic amino acids - Made from PEP! - From the Pentose Phosphate Pathway (an alternative to glycolysis) Sintesis Histidin Biosintesis Heme - In addition to proteins, some amino acids are used to make cofactors and signaling molecules: - Porphyrins, for example, are made from Succinyl CoA and Glycine Biosintesis Porfirin - The fundamental unit of porphyrins is -aminolevulinate (ALA) - Made by the pyroxidal phosphate (PLP) dependent enzyme aminolevulinate synthase PLP (vitamin B6) Biosintesis Porfirin - We then combine 2 ALA into Porphobilinogen Ring close via Schiff Base Biosintesis Porfirin dari PBG - Porphyrins are composed of 4 PBG subunits - The difference between Uroporphyrinogen I and III METABOLISME NUKLEOTIDA Metabolisme Nukleotida (nukleosida trifosfat) Nukleotida: Senyawa ester fosfat dari suatu gula pentosa dengan basa nitrogen yang terikat pada atom C1 dari pentosa Basa : Purin (Adenin, Guanin) ; Pirimidin (Urasil, Timin, Sitosin) Gula : Ribosa (RNA), Deoksi ribosa (DNA) Unit monomer yang berfungsi sebagai prekursor asam nukleat dan fungsi biokimia lainnya contoh : AMP, GMP, UMP, TMP, CMP Katabolisme Nukleotida Asam nukleat (DNA dan RNA) dari diet didegradasi menjadi nukleotida oleh nuklease pankreas dan fosfodiesterase usus halus Nukleotida didegradasi menjadi nukleosida oleh nukleotidase dan nukleosida fosfatase Nukleosida diserap langsung Degradasi lanjutan Nukleosida + H2O basa + ribosa (nukleosidase) Nukleosida + Pi basa + r-1-fosfate (n. fosforilase) Katabolisme Purin (Adenin dan Guanin): 90% digunakan kembali (salvage) (pada mamalia) 10% didegradasi menjadi asam urat Basa adenin → inosin → hipoksantin; adenosin deaminase, nukleosidase Asam urat pada beberapa jenis hewan didegradasi lebih lanjut Berbeda antar beberapa golongan hewan Asam urat → primata, burung, reptil, serangga Alantoin → mamalia lain Asam alantoat → ikan Urea → ikan bertulang rawan dan amfibi Amonia → invertebrata laut Katabolisme Pirimidin (Sitosin, Timin, Urasil): Reaksi : defosforilasilasi, deaminasi, dan pemutusan ikatan glikosida. Urasil dan timin direduksi di hati Produk akhir: ß-alanina (dari sitosin dan urasil) ß-aminoisobutirat (dari timin) Biosintesis Nukleotida Biosintesis purin (Adenin dan Guanin) o Jalur de novo → dari prekursor sederhana o Jalur salvage → dari hasil degradasinya Biosintesis Pirimidin (Sitosin, Urasil, dan Timin) Biosintesis Purin jalur de novo Diawali dengan sintesis IMP (Inosin MonoPhosphate) Terbuat dari 6 prekursor sederhana (CO2; Glisin; 2 Format; Glutamin; dan Aspartat) Sintesis IMP terdiri dari 11 tahapan reaksi 11 tahapan Reaksi Sintesis IMP 1. Aktivasi ribosa-5-fosfat 2. Penambahan glutamin → atom N9 3. Penambahan glisin → C4, C5, dan N7 4. Penambahan format → C8 5. Penambahan glutamin → N3 6. Pembentukan cincin imidazola 7. Penambahan bikarbonat → C6 8. Penambahan aspartat → N1 9. Eliminasi fumarat 10. Penambahan format → C2 11. Siklisasi IMP Sintesis AMP dan GMP 1. Adenilosuksinat sintase 2. Adenilosuksinase 3. IMP dehidrogenase 4. Transamidinase 1 IMP 3 AMPs XMP 2 4 AMP GMP Regulasi sintesis Purin Biosintesis Purin jalur salvage Penggunaan ulang hasil degradasi nukleotida menjadi nukleotida Memerlukan energi yang lebih rendah daripada sintesis de novo Memerlukan 2 enzim penting HGPRT (hipoksantin-guanin fosforibosil transferase) APRT (Adenin fosforibosil transferase) Jalur salvage Adenin Jalur salvage Guanin Biosintesis Pirimidin Diawali dengan sintesis UMP (Uridin MonoPhosphate) Terbuat dari 3 prekursor sederhana (HCO3-; Aspartat; dan glutamat) Sintesis UMP terdiri dari 6 tahapan reaksi Sintesis UTP Sintesis CTP E. coli Manusia dan hewan