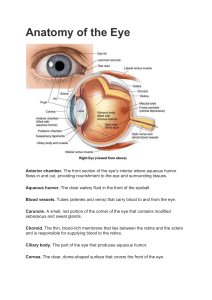
0736-5748/92 $5.00+0.00 Pergamon Press Ltd. © 1992 ISDN Int. J. Devl. Neuroscience, Vol. 10, No. 6, pp. 545-566, 1992 Printed in Great Britain. NEURONAL SPROUTING AND SYNAPSE FORMATION IN RESPONSE TO INJURY IN THE MOUSE ORGAN OF CORTI IN CULTURE HANNA M. SOBKOWICZ* and SUSAN M. SLAPNICK Department of Neurology, University of Wisconsin, Madison, WI, U.S.A. (Received 7 May 1992; in revised form 10 July 1992; accepted 13 July 1992) Abstract--The effect of mechanical injury on induction of regenerative phenomena within the neurosensory epithelium was investigated in cultures of neonatal mouse cochlea. The oldest examined culture in which new neuronal growth followed insult, was injured at 13 days in vitro and fixed 24 h later. By far, the most vigorous regenerative reaction was observed in a 3-day culture 4 h post-injury. The reaction included sprouting of nerve fibers injured directly, synapse formation between the surviving hair cells and sprouting neuronal growth cones, wrapping of growing nerve fibers by extending processes of hair cell cytoplasm, and collateral sprouting of synaptically-engaged nerve endings and of nerve fibers in passage. Key words: organ of Corti, mechanical injury, regeneration, hair cells, neuronal growth, synapse formation. The peripheral hearing organ of mammals is unique among sensory systems in its restricted number of receptors. It functions with about 481 sensory cells/mm: 103 inner hair cells and 378 outer hair cells. 3 The organ of Corti of the mouse operates using ca 3400 sensory cells in total; among them the number of primary receptors - - inner hair cells - - equals only 800. 4 Thus, each cell is precious. According to contemporary evidence, the mammalian sensory cells are formed once in a lifetime and their only fate is degeneration. Some cell loss, especially of outer hair cells, occurs spontaneously throughout the life span of an individual, whereas some is provoked by noise damage or drug toxicity, mainly as a side effect of amyloglycoside treatment. The last two factors are also most commonly used experimentally to induce sensory degeneration of the organ [see the review by Saunders e t al. (1985)]. 11In mammals, the lost hair cells are replaced by scars formed by supporting cells. 2'9 Thus, previous work concerned with injury to the neurosensory epithelium of the cochlea dealt mainly with the post-degenerative sequelae. Using mechanical trauma to injure hair cells, however, offers an alternative because, amazingly, many cells survive the initial insult and subsequently regenerate.14 Auditory hair cells receive double innervation: the locally present spiral ganglion neurons provide afferent innervation, whereas efferent innervation is derived from neurons located in the brain stem [see the review by Sobkowicz (1992)]. 13 The organ of Corti maintained in culture is innervated uniquely by the segment of spiral ganglion that has been excised with the corresponding cochlear turn. 15 The survival and maintenance of spiral ganglion cells is regulated by the sensory cells. In longterm experiments, the degeneration of hair cells results in a decrease of nerve cells. 28 The immediate reaction of spiral ganglion cells to the disappearance or injury of their target cells is less known. We shall present here the early ultrastructural response of afferent nerve endings to mechanical injury of the hair cell region. Our work centers mostly on the response elicited in a 3-day culture of a neonatal cochlea 4 h post-injury. The insult induced vigorous sprouting of nerve endings and synapse formation between hair cells and nerve growth cones. *Author to whom correspondence should be addressed at: University of Wisconsin, Neurology Department, Room 75 Medical Science Center, 1300 University Avenue, Madison, WI 53706, U.S.A. Abbreviations: bla, basal lamina; Bin, basilar membrane; C, Claudius' cells; Cp, cuticular plate; Cs, collagen substrate; Dc, Deiters' cell; Dg, degeneration; DIV, days in vitro; ex, extracellular matter; Ga, Golgi apparatus; Ih, inner hair cell; Ip, inner pillar; Iph, inner phalahgeal cell; Is, inner spiral sulcus cell; mt, microtubules; Nc, nerve growth cone; Ne, nerve ending; Nf, nerve fiber; Nu, nucleus; Oh, outer hair cell; Op, outer pillar; Os, outer spiral fibers; Oz, outgrowth zone; PI, (days) post-injury; Rm, Reissner's membrane; Sc, supporting cell; Sl, spiral limbus; Sn, spiral neuron; Sp, spiral prominence; St, satellite cell; Sv, stria vascularis; Tf, tunnel fiber; Tin, tectorial membrane; v, vesicles. oN Io:6-o 545 546 H . M . Sobkowicz and S. M. Slapnick EXPERIMENTAL PROCEDURES W e i n t r o d u c e d the t e c h n i q u e of t r a n s p l a n t i n g the m a m m a l i a n o r g a n of Corti into the c u l t u r e assembly a n d m a i n t a i n i n g it in vitro 15 in o r d e r to visualize a n d m a n i p u l a t e the d e v e l o p m e n t a l events in the cochlea. This biological m o d e l p r o v e d to be especially useful in defining the m u t u a l i n f l u e n c e of n e u r o s e n s o r y e l e m e n t s d u r i n g n o r m a l a n d e x p e r i m e n t a l c o n d i t i o n s , in defining the type of n e u r o n a l growth, a n d in eliciting r e g e n e r a t i v e responses of n e r v e fibers. ,~.~6,19T h e facility with which the o r g a n can be visualized, depicted a n d m a n i p u l a t e d in vitro m a k e s tissue culture the Fig. 1. A cross-section of an apical turn, 48 h after explantation. The explant rests with its basilar membrane on the collagen substrate. The black arrows indicate the direction of injury that aims either toward the basilar membrane or Reisner's membrane, depending on the position of the explant in relation to the substrate. (Reprinted with permission from Sobkowicz et al.. 1984.L8) Fig. 2. A cross-section of the hair cell region in the pre-injury area of a 3-day culture of a mid-turn. One inner and four outer hair cells are present, each accompanied by their respective supporting cells. Inner and outer pillars are the cells that will bound the future tunnel o f Corti. On the left, nerve fibers enter the organ to supply the inner and outer hair cells (in the three rows). Note that all nerve endings are confined to the lower poles of the hair cells. N e u r o n a l sprouting in injured organ of Corti Fig. 3. An overall view of a 3 DIV organ of Corti 4 h post-injury to the outer hair cell area. A: The inner hair cell with its nerve supply remains intact. 547 548 H . M . Sobkowicz and S. M. Slapnick Fig. 3B: The pulled-glass pipette has pierced the basilar membrane, destroying the Deiters' cells and outer hair cells in the second and third rows. The Oh-1 is spared, but its cuticular plate has been either shaved or smashed. Note the localized effect of the injury. Neuronal sprouting in injured organ of Corti 549 Fig. 3C: Under higher magnification, the injured outer hair cell reveals a denuded receptor pole with only one ending still attached. The injured nerve endings sprout and climb along the free side of the hair cell. One adjoins the apical surface of the cell. The arrow points to a presynaptic ribbon complex apposed to hair cell membrane facing a supporting cell: higher magnification in inset. technique of choice in experimental research on the developing organ. The protocol for maintaining the cultures is described in the initial publication by Sobkowicz et al. in 1975.15 The reaction of the sensory epithelium to mechanical injury was investigated in cultures of the organ of Corti from newborn H A / I C R mice (Harlan Sprague Dawley). Thirty-five cultures were injured by hand, using a pulled-glass pipette, between 45 h and 13 days in vitro (DIV), and allowed a post-injury (PI) recovery time from 4 h to 8 days. Each was then fixed for electron microscopy following the technique described by Guillery et al. 5 RESULTS We investigated ultrastructurally the regenerative response of the nerve endings of the peripheral fibers of spiral neurons to mechanical injury of the sensory epithelium. 550 H.M. Sobkowicz and S. M. Slapnick Fig. 3D: High magnification of the long sprouting filopodium climbing the free lateral surface of the surviving outer hair cell. Depending on the position of the explant on the substrate, the injury was made either through the basilar membrane (the floor of the organ) or through Reissner's membrane (the roof of the cochlear duct). Figure 1 shows the normal anatomical relationship in an explant of the organ of Corti excised from a newborn mouse in a 2-day culture. The black arrows indicate the directions in which the injury was aimed. Figure 2 shows the ultrastructure of cellular arrangements in the control, pre-injury area of an experimental culture at 3 DIV, that was fixed at 4 h PI. Each hair cell consists of an infranuclear neuroreceptor pole that serves as a synaptic anchor for nerve endings, an elongated supranuclear region that houses an elaborate Golgi apparatus, and a free apical part furnished with a cuticular plate and stereocilia; the youngest specimen examined and with the shortest time of fixation. Depending on the site of the entering pipette, either the free apical part or the infranuclear receptor pole of the hair cell is preferentially exposed to injury. In effect, both types of injury result in damage to the cuticular plate. It is possible that, when piercing the basilar membrane, the Neuronal sprouting in injured organ of Corti 551 Fig. 4. Ten microns away, another surviving Oh-1 shows a partly erased cuticular plate which is being replaced by cytoplasm (high magnification in Fig. 6). Injured nerve endings are again seen to sprout and climb along and atop the surviving hair cell. Double arrows in A and B point to the same sprouting nerve ending in the immediate area of the injury. High magnification of this growth cone in B shows multiple growth vesicles, an island of dense core vesicles (on the right), and a thin amorphous filopodium (arrow). free surface of the sensory epithelium is inadvertently p u s h e d against Reissner's m e m b r a n e or the stria vascularis which, facing the substrate, c a n n o t recoil. Direct injury to the cuticular plates of sensory cells sometimes spares the integrity of n e u r o s e n s o r y contacts (unless the process of repair p r e c e d e d the time o f fixation). T h e nerve fibers r e s p o n d with growth both to direct injury of their synaptic endings and to injury restricted to their hair cells. T h e oldest culture in which the regenerative response of nerve fibers o c c u r r e d was injured at 13 D I V and fixed 24 h later. O f the reactions observed, h o w e v e r , n o n e were c o m p a r a b l e in vigour to that d e m o n s t r a t e d in the 3 D I V , 4 h post-injury culture. 552 H.M. Sobkowicz and S. M. Slapnick Fig. 4B. This response was expressed by the sprouting of nerve endings evoked by direct injury, the formation of new synapses between the surviving hair cells and the neuronal growth cones, the wrapping of growing nerve fibers by extending processes of hair cell cytoplasm, and the collateral sprouting of synapticaUy-engaged nerve endings and of nerve fibers in passage. The response of nerve endings to direct injury Figures 3A and B illustrate the injured hair cell region in the culture shown in Fig. 2. The injuring pipette pierced the basilar membrane and partly destroyed the outer hair cell region, while sparing the inner hair cell with its nerve supply. Fortuitously enough, the outer hair cell in the first row also remained intact. Most of the synaptic endings that belonged to the surviving outer hair cell are eliminated, leaving its receptor pole free (Fig. 3C). New growth cones, however, are seen to grow away from the site of disintegration. They climb along the freed side of the hair cell membrane and reach its apical surface, denuded of cuticular plate (Figs 3C and D). Figure 4A illustrates a slightly different area of the injury site. The upper part of the surviving first row outer hair cell is compressed and contains some shrunken degenerative tissue. The cuticular plate is reduced to a remnant with only a few stereocilia remaining. Cytoplasm occupies most of the cell apex, and numerous neuronal growth cones collect in this area. Another outer hair cell, misplaced but still alive, is apposed to the lower pole of the first row of outer hair cells. Damaged disintegrating nerve endings mix with sprouting neuronal growth cones. Double arrows in Figs 4A and B point to the sprouting neuronal growth cone within the area of destruction. The ending, filled with membrane growth vesicles of different sizes, extends a long filopodium along the invading process of a supporting cell. In the intact organ, the free apical surface of hair cells is normally occupied by the cuticular plate and stereocilia. Laterally, the cuticular plates are bound by tight junctions to the supporting cells, forming a continuous reticular lamina, which normally is inaccessible to growing nerve fibers. Figures 3C, D, 4A, 6 and 8 suggest that after injury, in the absence of forbidding Neuronal sprouting in injured organ of Corti 553 Fig. 5. The same Oh-1 as that in Fig. 4A several sections away: a contrast between the disruption of neurosensory relationships at the site of injury and the preservation of synaptic contacts (arrows) at the untouched side of the receptor pole. supporting cells, regenerating nerve fibers reach the apical cell surface with predominant frequency. This trend suggests that the nerve growth cones are growing away from the injury. The effect of injury may be quite localized, selectively destroying some endings while sparing others. Despite the proximity to areas of destruction, the spared nerve endings appear to retain their synaptic connections. Figure 5 illustrates the receptor pole of an outer hair cell in the area. On the left-hand side, some nerve endings retain synaptic contacts marked by distinct ribbon synapses, 21 while the devastating results of the injury are evident in the adjoining area. Two large sprouting growth cones are prominent. The process of repair is also indicated by the presence of long processes of a supporting cell invading the region of damage. DN I0:6-E 554 H.M. Sobkowicz and S. M. Slapnick Fig. 6. High magnificationof the injured cuticular plate shown in Fig. 4A. A few stereocilia still remain; a group of growth cones invades the adjacent apical cytoplasmicsurface. The arrow points to the site of an incipient ribbon synapse (see inset) formed on contact with the ending. The basal body of the kinocilium lies just to the right of the cuticular plate (arrowhead). The formation of new synapses Apposition of the regenerating growth cones to an accessible hair cell m e m b r a n e frequently leads to the formation of new ribbon synapses. The ectopic location of these synaptic sites, at the apical surface of the hair cells, evidences their recent origin. Figures 6, 7 and 8 illustrate de novo formation of synaptic connections. In Figs 6 and 7, the apical portion of the cell is folded over the main cell body. On the left-hand side, the remnant of the cuticular plate and some stereocilia are visible. The newly growing nerve cones pile on top of the cytoplasmic portion of the cell apex. Two different ribbon synapses, at different intervals, are formed during the interaction between the m e m b r a n e s of nerve fibers and hair cells. Figure 8 shows a different regenerating outer hair cell forming a ribbon synapse with one of the growth cones adjoining its apical part. The synapsing cellular process of the hair cell extends forward to the neuronal growth cones. Formation of ectopic ribbons apposed to the hair cell m e m b r a n e apart from the neuroreceptor pole (Fig. 10) is characteristic of denervated hair cells. 2° In the absence of apposed nerve endings, the partly denervated hair cells may form ribbon presynaptic complexes across non-neuronal elements (Fig. 3C), TM In the present material, the tendency of the presynaptic complex to be formed on contact with the growth cones, regardless of the actual site, suggests the role that the latter may play in inducing the synaptic sites. In the apical cytoplasm of most outer hair cells surviving injury, the multitude of m e m b r a n e growth vesicles, a centriole and pericentriolar fibrous densities illustrate the ongoing process of cellular repair (Figs 6 and 7). 22 The wrapping of growing nerve fibers by hair cells In the young intact developing organ of Corti, nerve endings adjoining the hair cell receptor poles form fairly uncomplicated appositions (Fig. 5). In contrast, in the injured culture, we have occasionally noticed growing processes of hair cells extending to wrap the nerve growth cones. Figures 9-11 illustrate this unusual event. In Fig. 9, the cuticular plate of the first row outer hair Neuronal sprouting in injured organ of Corti 555 Fig. 7. The same Oh-1 (shown in Figs 4A and 6) several sections away: four growth cones adjoin the cytoplasmic portion of the cell's apex; the cell again responds by forming a ribbon presynaptic complex on contact with one of them (arrow). Note numerous membrane growth vesiclesin the apical portion of the hair cell. cell is gone. Some neuronal growth cones adjoin the apical cell surface while the hair cell itself wraps around an ectopic nerve fiber. Figure 10 shows the apical surface of one of the traumatized outer hair cells in the first row: on the left-hand side is a remnant of the cuticular plate with three stereocilia; cytoplasm occupies the right-hand part. A very active Golgi apparatus and myriad m e m b r a n e growth vesicles reaching up to the top depict active regenerative p h e n o m e n a . Apposing its top is the cytoplasmic process of a neighboring cell (see Oh-2 in Fig. 8) clasping a nerve fiber. Figure 11 shows the hair cell process firmly enclosing the nerve fiber while itself becoming anchored to the cuticular plate of its neighbor by a specialized junction. The hair cell process displays m a n y m e m b r a n e vesicles and microtubules. In both figures, successive nerve growth cones have arrived on top of it. Thus, mechanical injury induces new growth both in nerve fibers a n d - - m o s t unusually--in the hair cells themselves. 556 H.M. Sobkowicz and S. M. Slapnick Fig. 8. A new ribbon synapse formed with a neuronal growth cone at the cell apical surface (double arrow). Both growth endings are filled with vesicles, some of synaptic size (single arrow). Notice a cytoplasmic process (asterisk) of Oh-2 which subsequently wraps one of the growing nerve fibers in the area (Fig. 10). Collateral sprouting of nerve endings Collateral sprouting is a term usually reserved for a growth sprouting from uninjured endings as a response to injury of other nerve fibers in the immediate neighborhood [see the review by kiu, (1981)].s As an outcome, the sprouting growth cones occupy the vacant synaptic sites left by the injured nerve fibers. This last characteristic does not apply to our experimental situation. The sprouting of nerve endings in the injured cultures occurred within the nerve supply to inner hair cells, when the injury afflicted only the outer hair cell region. The area of the inner hair cells and their endings appears to be physically unharmed as are the supporting cells. A m o n g the latter, the inner and outer pillars normally form a substantial barrier between the outer and inner hair cell regions. The newly growing nerve endings invade the territory of their own receptors. The sprouting filopodia evidently exert considerable force, deeply invaginating the hair cell cytoplasm opposed only by the mass of the nucleus. Figures 12 and 13 illustrate sprouting nerve endings distant to the injury. In Figs 12A and B the nerve ending itself indicates signs of growth: bulging and filled with vesicles, microtubules and microfilaments, it flows beneath the inner hair cell and sprouts two stout filopodia into the territory of its receptor. In Figs 13A and B the growing ending is synapticatlyengaged with the inner hair cell; regardless, the ending sprouts at least five filopodia that invaginate the hair cell cytoplasm up to the nucleus. Some of the filopodia evoke synaptic contacts (Fig. 14). Neuronal sprouting in injured organ of Corti 557 Fig. 9. An outer hair cell wraps itself around an ectopic nerve fiber. Its cuticularplate is gone; a cluster of growth cones adjoins the apical cell surface. Collateral sprouting of apparently uninjured nerve fibers also may be observed among fibers en p a s s a g e . One example (Fig. 15) displays a tunnel fiber that evidently reversed its usual direction of growth, sprouting back toward the inner hair cell and away from the injury. DISCUSSION Our results illustrate the early reparative efforts of hair cells and their nerve endings induced by recent mechanical trauma. The regenerative phenomena include (1) sprouting of nerve fibers, not only of those injured directly, but also of those distant to the site of injury, and (2) an attempt to reinnervate, expressed by the formation of new synapses between hair cells and the proliferating growth cones. Most of our injured cultures presented some evidence of neuronal growth among the endings supplying the injured or recovering hair cells. None, however, provided us with such forceful events of new growth as the one fixed earliest, i.e. at 4 h post-injury. The time of fixation was chosen arbitrarily, and it may be that in cultures fixed at later times, the neuronal repair was mostly completed and new synaptic contacts already achieved. Few surviving but denervated hair cells were observed. It has to be stressed that the described regenerative phenomena occurred in the developing organ of Corti. In the intact animal, large growing nerve endings are commonly seen in 1-day postnatal animals and, in culture, nerve growth cones may occasionally be seen up to 6 DIV. TM Afferent synaptogenesis of inner hair cells both in the intact animal and in culture continues for about 5 days postnatally, and the number of ribbon synapses in the outer hair cells increases for about 6-8 days. z° Thus, the illustrated growth phenomena are induced in hair cells and spiral neurons during their normal development. 558 lq. M. S o b k o w i c z a n d S. M. S t a p n i c k Fig. 1~. T h e double arrow points to a cellular process of an outer hair cell from the second row (Fig. 8, asterisk) clasping a nerve fiber (arrowheads). This unusual formation rests on the upper portion of the first row outer hair cell near its cuticular plate. Nerve growth cones piling atop are characterized by large irregular m e m b r a n e vesicles, microtubules and dense core vesicles. Two ribbon-like densities adjoin the hair cell m e m b r a n e facing the cuticular plate (arrows). Conspicuous m e m b r a n e growth vesicles and a Golgi apparatus extending to the surface of the cell reflect active growth within the hair cell cytoplasm. N e u r o n a l sprouting in injured o r g a n of Corti Fig. 11. Another example of a hair cell wrapping a neuronal process (double arrow). The nerve fiber is firmly embraced by the hair cell process which itself is attached by a specialized junction (arrow) to the Oh-1 cuticular plate. The wrapping process is filled with membrane growth vesicles and mitochondria. Nerve growth cones decorate its upper surface. 559 560 H . M . Sobkowicz and S. M. Slapnick Fig. 12. Collateral sprouting of nerve fibers distant to the injury. A and B show two fairly close sections of a sprouting nerve ending beneath an inner hair cell. Two stout filopodia invaginate the hair cell cytoplasm (arrows), stopped only by the mass of the nucleus. Growth vesicles and dense core vesicles (arrowheads) characterize the growth. Note an extracellular dense material. The inset in A shows a higher magnification of a synaptic vesicle forming a presynaptic complex across a supporting cell cytoplasm (right arrowhead in the main picture). Neuronal sprouting in injured organ of Corti Fig. 12B. 561 Fig. 13, Distant sections showing lilopodia (arrows) sprouting from the synaptically-engaged nerve ending of a neighboring inner hair cell. A: Arrowheads point to growth vesicles. B: The double arrow points to the site where the filopodium touches the nuclear membrane. The arrowhead points to a ribbon synapse, =_. Oq, © O O" "r J Neuronal sprouting in injured organ of Corti 563 Fig. 14. Growth cone and two filopodia in cross-sections (asterisks) in contact with an inner hair cell. Arrows point to ribbon synapses with the main fiber as well as with the left filopodium. A group of synaptic vesicles clustering at the plasma membrane is indicated with a 'v'. The ability of nerve fibers to regenerate following damage or destruction of sensory cells is not limited to the developing organ. The presence of regenerative nerve fibers in the adult m a m m a l i a n cochlea has been observed both in noise-exposed and in antibiotic-treated animals [for a review, see B o h n e and Harding (1992)]. 1 Regenerative p h e n o m e n a were expressed by sprouting of multiple processes from surviving spiral neurons which thus became multipolar, 27 by the presence of proliferating nerve fibers within the area of sensory destruction, 7'3° by a m a r k e d proliferation of end collaterals of the radial fibers after transection of the V I I I nerve, ~3 and by new growth and myelination of nerve fibers, interpreted to be efferent. 24'25 Bohne and Harding, 1 who recently reinvestigated the regenerative response of nerve fibers induced by noise damage to the cochlea, suggest that the p h e n o m e n o n is widespread and was possibly underrated previously. The different techniques used in damaging the organ of Corti do not permit one to c o m p a r e the times of the onset of the regenerative response in the adult cochlea. The newly growing nerve fibers may be seen any time from 1 week 24'25 to 1-3 months 1 to 20-24 months in the case of V I I I nerve transection, z3 Evidently the young age of our specimen may be a chief factor responsible for the rapid 4 h response and new growth. T h e r e are still some basic questions to ask in future experiments. H o w does increasing age modify the onset of neuronal sprouting? H o w long does the new growth last, and what are the conditions for neuronal sustenance and regeneration? The regulatory influence of hair cells on the growth of nerve fibers was first described in cultures of the organ of Corti where, during normal development and under experimental conditions,I° the presence and position of hair cells define the length of the innervating fibers and distribution of their endings; whereas the total disruption of synaptic contacts and the elimination of hair cells result in a continuous but disorganized free growth of nerve fibers. The surviving sensory cells a p p e a r also to play a role in the redistribution of nerve endings in the developing deaf Bronx waltzer mouse, both in the intact animal 29 and in culture. 17 Subse- 564 H.M. Sobkowicz and S. M. Slapnick 4 Fig. 15. A bulbous growth cone from a tunnel fiber sprouting in an area distant to the injury. This fiber is growing in the reverse direction; awayfrom the injured hair cell and toward the inner hair cell. Note the dense core vesicles (arrows), microtubules and numerous growth vesicles. quently, those nerve cells in the mutant that manage to sustain or to acquire new synaptic connections with the remaining receptors show long-term survival, while others evidently perish. 6"12 The influence of nerve fibers on the maintenance or differentiation of hair ceils is less known. Van de Water observed in culture survival and advanced differentiation of the sensory epithelium of the mouse otocyst after an early extirpation of the statoacoustic ganglion. 26 Sobkowicz e t al. 2° maintained denervated organ of Corti for 14 days in culture. In the absence of nerve fibers, the hair cells adjoined only by supporting cells formed presynaptic ribbon complexes apposed to the hair cell membrane but distributed at random. A high incidence of annulate lameUae in denervated hair cells TM suggests a metabolic imbalance (produced by a lack of nerve fiber supply). Neuronal sprouting in injured organ of Corti 565 A m o n g the d e s c r i b e d p o s t - i n j u r y p h e n o m e n a , two are u n i q u e . O n e is the a p p a r e n t growth of n e u r o n a l cones away f r o m the i n j u r y / d e g e n e r a t i o n site. T h e o t h e r is the u n u s u a l w r a p p i n g of n e r v e growth cones by e x t e n d i n g cytoplasmic processes of hair cells. U n d e r n o r m a l c o n d i t i o n s , g r o w i n g n e r v e e n d i n g s p r e f e r e n t i a l l y appose the hair cell pole at the peri- a n d i n f r a n u c l e a r levels. It is n o t u n u s u a l to o b s e r v e e x p l o r a t o r y n e r v e e n d i n g s c l i m b i n g a l o n g hair cell bodies, b u t the f o r m a t i o n of afferent r i b b o n synapses in the vicinity of the cuticular plate is very rare. W e c a n n o t offer an e x p l a n a t i o n for this u n u s u a l e v e n t o t h e r t h a n to suspect the adverse directive effects of the d e g e n e r a t i v e debris in the i n j u r y area. T h e e x t e n d i n g cytoplasmic processes of hair cells that seek i n t i m a t e m e m b r a n e c o n t a c t with n e r v e e n d i n g s are m o s t u n u s u a l . N o r m a l l y , growing n e r v e fibers within the sensory o r g a n o b t a i n s u p p o r t o n l y f r o m the s u p p o r t i n g cells in the area, i.e. f r o m the i n n e r spiral sulcus cells, b o r d e r cells of H e l d , pillar cells a n d D e i t e r s ' cells. (In the i n j u r e d cultures, hair cells t h e m s e l v e s m a y be w r a p p e d by the same s u p p o r t i n g cells.) TM F u t u r e studies will shed light o n this n e w i n t e r c e l l u l a r r e l a t i o n s h i p b e t w e e n the s e n s o r y cells a n d their n e r v e fibers. CONCLUSION A p p l y i n g m e c h a n i c a l t r a u m a as a m e a n s of i n j u r i n g the n e u r o s e n s o r y e p i t h e l i u m p e r m i t s us to study the i n d u c t i o n of r e g e n e r a t i v e m e c h a n i s m s in b o t h s e n s o r y a n d n e r v e cells. T h e early r e g e n e r a t i v e r e s p o n s e of n e r v e fibers a p p e a r s to be i n f l u e n c e d by the surviving receptor cells r a t h e r t h a n by their deficit. Acknowledgements This work was supported by NIH grant DC00517. We are grateful to Ms Amy McDaniel for editing the manuscript and preparing the illustrations. REFERENCES 1. Bohne B. A. and Harding G. W. (1992) Neural regeneration in the noise-damaged chinchilla cochlea. Laryngoscope, in press. 2. Bohne B. A. and Rabbit K. D. (1983) Holes in the reticular lamina after noise exposure: Implications for continuing damage in the organ of Corti. Hearing Res. 11, 41-53. 3. Burda H., Ballast L. and Bruns V. (1988) Cochlea in old world mice and rats (Muridae). J. Morphol. 198, 269-285. 4. Burda H. and Branis M. (1988) Postnatal development of the organ of Corti in the wild house mouse, laboratory mouse, and their hybrid. Hearing Res. 36, 97-106. 5. Guillery R. W., Sobkowiez H. M. and Scott G. L. (1970) Relationships between glial and neuronal elements in the development of long term cultures of the spinal cord of the fetal mouse. J. comp. Neurol. 140, 1-34. 6. Keithley E. M. and Feldman M. L. (1983) The spiral ganglion and hair cells of Bronx waltzer mice. Hearing Res. 12, 381-391. 7. Lira D. J. (1976) Uitrastructural cochlear changes following acoustic hyperstimulation and ototoxicity. Ann. Otol. 85, 740-751. 8. Liu H. M. (1981) Biology and Pathology of Nerve Growth. Academic Press, New York. 9. Raphael Y. and Altschuler R. A. (1991) Scar formation after drug-induced cochlear insult. Hearing Res. 51, 173-184. 10. Rose J. E., Sobkowicz H. M. and Bereman B. (1977) Growth in culture of the peripheral axons of the spiral neurons in response to displacement of the receptors. J. Neurocytol, 6, 49-70. 11. Saunders J. C., DearS. P. and Schneider M. E. (1985) The anatomical consequences of acoustic injury: A review and tutorial. J. acoust. Soc. Amer. 78, 833--860. 12. Schrott A., Stephan K. and Spoendlin H. (1989)Hearing with selective inner hair cell loss. Hearing Res. 40, 213-220. 13. Sobkowicz H. M. (1992) The development of innervation in the organ of Corti. In Development of Auditory and Vestibular Systems H, (ed. Romand R.), pp. 59-100, Elsevier, Amsterdam. 14. Sobkowicz H. M., August B. K. and Slapnick S. M. (1992) Epithelial repair following mechanical injury of the developing organ of Corti in culture: an electron microscopic and autoradiographic study. Expl Neurol. 115, 44-49. 15. Sohkowicz H. M., Bereman B. and Rose J. E. (1975) Organotypic development of the organ of Corti in culture. J. Neurocytol. 4, 543-572. 16. Sobkowicz H. M., Emmerling M. R. and Whitlon D. S. (1988) Development of cochlear efferents in the postnatal mouse. Abst. Assoc. Res. Otolaryngol. 11, 131. 17. Sobkowicz H. M., Rose J. E., Levenick C. V. and Scott G. L. (1988) Morphology of the cochlea in the Bronx waltzer mouse. Abst. Assoc. Res. OtolaryngoL 11, 149-150. 18. Sobkowicz H. M., Rose J. E., Scott G. L. and Holy J. M. (1984) The ultrastructure of the developing organ of Corti of the mouse in culture. In Ultrastructural Atlas of the Inner Ear (ed. Friedmann I. and Ballantyne J.), pp. 61-97. Butterworths, London. 19. Sobkowicz H. M., Rose J. E., Scott G. L., Kuwada S,, Hind J. E., Oertel D. and Slapnick S. M. (1980) Neuronal growth in the organ of Corti in culture. In Tissue Culture in Neurobiology, (ed. Giacobini E., Vernadakis A. and Shahar A.), pp. 253-275. Raven Press, New York. 20. Sobkowicz H. M., Rose J. E., Scott G. L. and Levenick C. V. (1986) Distribution of synaptic ribbons in the developing organ of Corti. J. NeurocytoL 15, 693-714. 566 H. M. Sobkowicz and S. M. Slapnick 21. Sobkowicz H. M., Rose J. E., Scott G. L. and Slapnick S. M. (1982) Ribbon synapses in the developing intact and cultured organ of Corti in the mouse. J. Neurosci. 2, 942-957. 22. Sobkowicz H. M. and Slapnick S. M. (1992) Morphogenetic role of the kinocilium in auditory hair cells. Conference on the Molecular Biology of Hearing and Deafness, La Jolla, CA. 23. Spoendlin H. and Surer R. (1976) Regeneration in the VIII nerve. Acta Otolaryngol. 81,228--236. 24. Terayama Y., Kaneko Y., Kawamoto K. and Sakai N. (1977) Ultrastructural changes of the nerve elements following disruption of the organ of Corti. I. Nerve elements in the organ of Corti. Acta Otolaryngol. 83, 291-302. 25. Terayama Y., Kaneko K., Tanaka K. and Kawamoto K. (1979) Ultrastructural changes of the nerve elements following disruption of the organ of Corti. II. Nerve elements outside the organ of Corti. Acta Otolaryngol. 88, 27-36. 26. Van de Water T. R. (1976) Effects of removal of the statoacoustic ganglion complex upon the growing otocyst. Ann. Otol. Rhinol. Laryngol. 85, 1-32. 27, Webster D. B. and Webster M. (1982) Multipolar spiral ganglion neurons following organ of Corti loss. Brain Res. 244, 356-359. 28. Webster M. and Webster D. B. (1981) Spiral ganglion neuron loss following organ of Corti loss: A quantitative study. Brain Res. 212, 17-30. 29. Whitlon D. S. and Sobkowicz H. M. (1991) Patterns of hair cell survival and innervation in the cochlea of the Bronx waltzer mouse. J. Neurocytol. 20, 886-901. 30. Wright C, G. (1976) Neural damage in the guinea pig cochlea after noise exposure. Acta Otolaryngol. 82, 82-94.