Kimia Karbon dan Polimer
advertisement

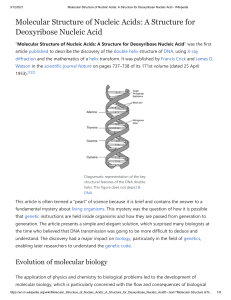
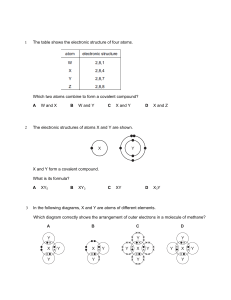
Kimia Karbon dan Polimer 1. Hidrokarbon 2. Gugus fungsi Karbon 3. Organo kimia 4. Polimer Kimia karbon Dalam sistem kehidupan 70-90% air danseimbang dengan carbon(C) Carbon is unparalleled in its ability to form molecules that are large, complex and diverse Atomic Structure of Carbon Key is the atom…nucleus and electrons Carbon has 6 electrons 2 in first shell 4 in next shell (for orbits, one electron per orbit) Little tendency to gain or loose electrons…no ionic bonding Greater likelihood for electrons to be shared with other atoms in covalent bonds…four (4) possible covalent bonds per atom of carbon Unsur kebanyakan berikatan dengan C C Oxygen Hydrogen Nitrogen Carbon Gugus fungsi Distinctive features of large carbon compounds depend on two aspects of the molecule Carbon skeleton (chained, branched, etc.) Groups of atoms that are attached to the carbon (called “functional groups”) 1. 2. 3. 4. 5. 6. hydroxyl (OH) carbonyl (CO) carboxyl (COOH) amino (NH2) sulphydryl (SH) phosphate (P) Organo kimia Pestisida, insektisida, herbisida • Senyawa Klor organik • Senyawa Posfor organik • Senyawa Karbamat, dll Polymer Principles Any long chain of repeating units is called a polymer (chemistry chapter)…key point is repeating units (train car analogy) Dalam Biokimia, phenomena ini dibagi dalam 4 klas senyawa yang betul-betul penting, 1. 2. 3. 4. Carbohydrates Proteins Nucleic acids Lipids In biology, the long linkages among carbon atoms are made via covalent bonds (weak bonds that allow for variety of transformations, including release of energy and ability to be spilt easily e.g., nerve impulse of a ringing of the bell) Carbohydrates Keys: (i) structure of basic unit and role of carbon, (ii) polymer structure, (iii) function, and (iv) structure and therefore function is inherited General description of fuel and building material for cells with a general formulation of strings of carbon bonded covalently into large macromolecules Repeated units (glucose) can be strung together in an infinite array (configured) to produce a large number of different chemicals each with different biochemical properties but each sharing the carbon skeleton, functional groups…hydroxyl and carboxyl) and the importance of covalent bonds…and all structure is genetically determined Basic unit structure (sugar) Polymer Structure (bonding of units) Function…structure and storage Inheritance Lipids Keys: (i) structure of basic unit and role of carbon, (ii) polymer structure, (iii) function, and (iv) structure and therefore function is inherited Very diverse macromolecules with one trait: little or no affinity for water (define hydrophobic) since molecular structure is mostly hydrocarbons Three types: fats, phospholipids and steroids Fats Structure chain of carbons (16-18 in length) – fatty acid Satuan gugus fungsi gliserol (alcohol) variation: number of carbons and double versus single C-C bonds OH OH -C-C-C-C-C-C-C-C-C-C-C OH Fatty Acid Glycerol (hydrophobic) (hydrophilic) Amino Acids and Proteins Function: Storage Structure Transport Enzymes (catalysis…selectively accelerates rates of reactions) Defense 10,000+ proteins, each with a unique structure and unique function Structure Most structurally sophisticated molecules Vary extensively in shape-…unique s-D shape Although diverse, all are polymers on 20 amino acids called polypeptides Protein definition: 1 or more polypeptides folded together…distinction is that a protein has a specific functional role 1. Polymers of a basic unit (amino acid) 2. Linked together to form a macromolecule via polypeptide bonds that are covalent 3. Unique 3-D shape that results in a functional protein 4. Very slight changes in conformation (primary to quaternary) can change the protein from one that is functional to one that is nonfunctional 5. Sequence of amino acids is genetically determined Nucleic Acids and DNA Theme of living systems is the feature of continuity such that information and directions are passed along from generation to generation, and this same feature is what determines that macromolecules are genetically inherited as well This information rich source is based on nucleic acids and the macromolecule that nucleic acids form, which is DNA Two types of nucleic acid macromolecules DNA – deoxyribonucleic acid RNA – ribonucleic acid Function of nucleic acids and their macromolecules Reproduction or “replication” Directs the synthesis of RNA which in turn controls the synthesis of proteins (“transcription” and “translation”)