Uploaded by
vhartanto20
University Lab Report: Carbohydrates in Cassava Starch Analysis
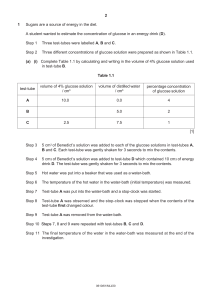
advertisement

LAPORAN RESMI PRAKTIKUM DASAR TEKNIK KIMIA II Materi : KARBOHIDRAT Disusun Oleh : 7-Senin Group : 7 - Senin Rekan Kerja : 1. Aurellia Livia Hidayat NIM. 21030120130110 2. Desita Rachmawanti NIM. 21030120120011 3. Ergian Janitra NIM. 21030120130103 4. Ghea Fsyifa Hidawati NIM. 21030120120025 LABORATORIUM DASAR TEKNIK KIMIA DEPARTEMEN TEKNIK KIMIA FAKULTAS TEKNIK UNIVERSITAS DIPONEGORO SEMARANG 2021 LEMBAR PENGESAHAN PRAKTIKUM DASAR TEKNIK KIMIA II UNIVERSITAS DIPONEGORO Materi : Karbohidrat Kelompok : 7 - Senin Anggota : 1. Aurellia Livia Hidayat NIM. 21030120130110 2. Desita Rachmawanti NIM. 21030120120011 3. Ergian Janitra NIM. 21030120130103 4. Ghea Fsyifa Hidawati NIM. 21030120120025 Semarang, Mengetahui Dosen Pengampu Asisten Pembimbing Ir. Kristinah Haryani, M.T. Vincent Hartanto NIP. 196402141991022002 NIM. 21030118130144 ii RINGKASAN Dalam kehidupan sehari-hari kita sering melakukan aktivitas yang membutuhkan energi cukup banyak sehingga memerlukan asupan makanan. Bahan makanan mengandung karbohidrat yang memegang peran penting dan sebagai sumber energi utama. Tujuan praktikum kali ini adalah membuat rangkaian alat analisa karbohidrat dan mengoperasikannya, menentukan reaksi-reaksi pada uji penentuan kadar pati, dan menentukan kadar karbohidrat (pati) pada tepung singkong. Karbohidrat adalah polisakarida aldehid/keton yang mempunyai rumus (CH2O)n. Karbohidrat dapat digolongkan menjadi 3, yaitu monosakarida, disakarida, dan polisakarida. Monosakarida merupakan karbohidrat yang paling sederhana, contohnya glukosa, galaktosa, fruktosa, dan ribosa. Disakarida merupakan karbohidrat yang terdiri dari dua satuan monosakarida, contohnya sukrosa (gabungan glukosa dan fruktosa), maltosa (gabungan dari dua unit glukosa), dan laktosa (gabungan glukosa dan galaktosa). Polisakarida merupakan karbohidrat yang terdiri dari banyak satuan (lebih dari delapan satuan) monosakarida, contohnya pati, selulosa, pektin, kitin, dan lain-lain. Pada praktikum ini, kami menggunakan bahan yaitu tepung singkong sebagai sampel, HCl 37%, NaOH 0,5 N, aquadest, glukosa anhidris 0,0025 gr/ml, metilen blue, fehling A, dan fehling B. Alat yang digunakan adalah rangkaian alat yang terdiri dari magnetic stirrer plus heater, waterbath, labu leher tiga, thermometer, pendingin balik, klem dan statif yang dirangkai sedemikian rupa, timbangan, buret, pipet volume, pipet tetes, gelas ukur, oven, kompor listrik, erlenmeyer, beaker glass, cawan porselin, corong, dan indikator pH. Prosedur kerja yang dilakukan yaitu analisa kadar pati dengan persiapan bahan untuk mengetahui densitas tepung singkong, standarisasi larutan Fehling dengan larutan glukosa standart, dan penentuan kadar pati pada tepung singkong. Pada praktikum ini, ditemukan kadar pati tepung singkong praktis sebesar 81,45% dan kadar pati tepung singkong acuan dari jurnal sebesar 85,045%. Penyebab dari kadar praktis lebih kecil dari kadar teoritis adalah sifat fisikokimia pati, kelarutan, dan konsentrasi asam di dalam larutan pada saat hidrolisa terlalu berlebihan. Saran-saran yang diberikan dari praktikum ini adalah Larutan Fehling dijaga agar tidak terkontaminasi dan HCl dijada agar tidak menguap, kecepatan magnetic stirrer diperhatikan agar tidak menimbulkan pusaran (vortex), dan titrasi harus dilakukan di atas kompor listrik untuk menjaga suhu larutan yang dititrasi agar konstan. iii PRAKATA Puji syukur kehadirat Tuhan Yang Maha Esa yang telah memberikan rahmat dan hidayah-Nya sehingga laporan Praktikum Dasar Teknik Kimia II ini dapat diselesaikan dengan lancar dan sesuai dengan harapan. Laporan praktikum ini diperuntukkan untuk memenuhi salah satu tugas mata kuliah Praktikum Dasar Teknik Kimia II. Adapun isi laporan praktikum ini adalah pembahasan mengenai hasil percobaan dari praktikum Karbohidrat. Berbagai dukungan dan doa sehingga penyusun dapat menyelesaikan laporan praktikum ini. Untuk itu, tim penyusun mengucapkan terima kasih kepada : 1. Dr. Ing. Ir. Silviana, S.T., M.T., IPM., ASEAN Eng. selaku dosen penanggung jawab Laboratorium Dasar Teknik Kimia II. 2. Ir. Kristinah Haryani, M.T. selaku dosen pengampu materi Karbohidrat. 3. Andrew Reynaldo Kristianto Handoko selaku koordinator asisten Laboratorium Dasar Teknik Kimia II. 4. Andrew Christian Timothy Prasetyo, Vincent Hartanto, dan Maria Asel selaku asisten pengampu materi Karbohidrat. 5. Asisten-asisten Laboratorium Dasar Teknik Kimia II lainnya. Kritik dan saran dari pembaca sangat diharapkan untuk penyempurnaan laporan praktikum ini karena masih banyak kekurangan yang perlu diperbaiki. Akhir kata, semoga laporan praktikum ini dapat bermanfaat sebagai bahan penambah ilmu pengetahuan. Semarang, 14 Maret 2021 Tim Penyusun iv DAFTAR ISI HALAMAN JUDUL ................................................................................................ i LEMBAR PENGESAHAN ..................................................................................... ii RINGKASAN ......................................................................................................... iii PRAKATA ............................................................................................................. iv DAFTAR ISI ........................................................................................................... v DAFTAR TABEL .................................................................................................. vi DAFTAR GAMBAR ............................................................................................. vii DAFTAR LAMPIRAN ........................................................................................ viii BAB I PENDAHULUAN ........................................................................................ 1 1.1 Latar Belakang............................................................................................ 1 1.2 Tujuan Praktikum ....................................................................................... 1 1.3 Manfaat Praktikum ..................................................................................... 2 BAB II TINJAUAN PUSTAKA ............................................................................. 3 2.1 Pengertian Karbohidrat ............................................................................... 3 2.2 Pati ............................................................................................................. 3 2.3 Hidrolisa Pati .............................................................................................. 5 2.4 Faktor-Faktor yang Mempengaruhi Hidrolisa .............................................. 6 2.5 Aplikasi Pati di Bidang Industri .................................................................. 7 BAB III METODE PRAKTIKUM......................................................................... 9 3.1 Alat dan Bahan yang Digunakan ................................................................. 9 3.1.1 Bahan .................................................................................................. 9 3.1.2 Alat ..................................................................................................... 9 3.2 Prosedur Praktikum .................................................................................. 10 1. Analisa Kadar Pati .................................................................................... 10 2. Pembuatan larutan fehling......................................................................... 11 3. Pembuatan Larutan Glukosa standart ........................................................ 12 BAB IV HASIL DAN PEMBAHASAN................................................................ 13 4.1 Perbandingan Kadar Praktis dan Teoritis................................................... 13 4.2 Mekanisme Hidrolisa Pati dengan Katalis Asam ....................................... 14 4.3 Mekanisme Penentuan Kadar Pati dengan Uji Fehling .............................. 15 BAB V PENUTUP ................................................................................................ 17 5.1 Kesimpulan............................................................................................... 17 5.2 Saran ........................................................................................................ 17 DAFTAR PUSTAKA ............................................................................................ 18 v DAFTAR TABEL Tabel 4.1 Perbandingan kadar pati tepung singkong acuan dengan kadar pati tepung singkong praktis ...................................................................................................... 13 vi DAFTAR GAMBAR Gambar 2.1 Struktur amilosa ..................................................................................... 4 Gambar 2.2 Struktur amilopektin .............................................................................. 4 Gambar 3.1 Rangkaian alat ..................................................................................... 10 Gambar 4.1 Mekanisme Hidrolisis Pati dengan Katalis Asam (Hoover, 2000) ......... 15 vii DAFTAR LAMPIRAN LAPORAN SEMENTARA................................................................................... A-1 LEMBAR PERHITUNGAN ................................................................................. B-1 LEMBAR KUANTITAS REAGEN ...................................................................... C-1 LEMBAR PERHITUNGAN REAGEN ................................................................ D-1 REFERENSI .......................................................................................................... E-1 LEMBAR ASISTENSI viii BAB I PENDAHULUAN 1.1 Latar Belakang Dalam kehidupan sehari-hari kita sering melakukan aktivitas yang membutuhkan energi cukup banyak. Energi ini kita peroleh dari bahan makanan yang kita makan. Pada umumnya bahan makanan itu mengandung tiga kelompok utama senyawa kimia yaitu karbohidrat, protein dan lemak. Karbohidrat memegang peranan yang sangat penting di alam karena merupakan sumber energi utama bagi manusia dan hewan. Kita dapat mengenal berbagai jenis karbohidrat dalam kehidupan sehari-hari contohnya amilum atau pati, selulosa, glikogen, gula atau sukrosa, yang berfungsi sebagai pembangun struktur maupun yang berperan fungsional dalam proses metabolisme. Pati merupakan karbohidrat utama dalam makanan yang berasal dari tumbuh-tumbuhan. Pati merupakan polisakarida yang diproduksi selama proses fotosintesis. Energi matahari diubah menjadi energi kimia dengan menggabungkan karbon dioksida dengan air untuk membentuk karbohidrat sederhana (glukosa) dan oksigen molekuler. Pati umumnya ditemukan pada umbi-umbian, biji-bijian, kacang-kacangan dan buahbuahan. Pada metabolisme manusia, karbohidrat kompleks perlu diubah menjadi bentuk yang lebih sederhana dengan bantuan enzim agar bisa dicerna oleh tubuh untuk menghasilkan energi. Dalam proses pencernaan semua bentuk pati dihidrolisis menjadi glukosa. Pada tahap petengahan akan dihasilkan dekstin dan maltosa. Dekstrin, merupakan produk antara pada pencernaan pati atau dibentuk melalui hidrolisis parsial pati. Glikogen, dinamakan juga pati hewan karena merupakan bentuk simpanan karbohidat di dalam tubuh manusia dan hewan, yang terutama terdapat di dalam hati dan otot. Glikogen dalam otot hanya dapat digunakan untuk keperluan energi di dalam otot tersebut, sedangkan glikogen dalam hati dapat digunakan sebagai sumber energi untuk keperluan semua sel tubuh. 1.2 Tujuan Praktikum 1. Membuat rangkaian alat analisa karbohidrat dan mengoperasikannya. 2. Menentukan reaksi-reaksi pada uji penentuan kadar pati. 3. Menentukan kadar karbohidrat (pati) pada tepung singkong dengan prosedur yang benar. 1 1.3 Manfaat Praktikum 1. Mahsiswa mampu menyusun rangkaian alat analisa karbohidrat dan mengoperasikannya. 2. Mahasiswa mampu memahami reaksi-reaksi pada uji penentuan kadar pati. 3. Mahasiswa mampu menentukan kadar karbohidrat (pati) pada tepung singkong dengan prosedur yang benar. 2 BAB II TINJAUAN PUSTAKA 2.1 Pengertian Karbohidrat Karbohidrat merupakan salah satu senyawa organik yang banyak dijumpai di alam yang mengandung atom karbon (C), hidrogen (H), dan oksigen (O). Rumus umum dari senyawa karbohidrat adalah (CH 2O)n. Senyawa karbohidrat merupakan polihidroksi aldehid dan keton atau turunannya. Berdasarkan ukuran molekulnya, karbohidrat diklasifikasikan dalam tiga golongan, yaitu monosakarida, disakarida, dan polisakarida. Monosakarida merupakan karbohidrat yang paling sederhana, contohnya glukosa, galaktosa, fruktosa, dan ribosa. Disakarida merupakan karbohidrat yang terdiri dari dua satuan monosakarida. Ada 3 isomer penting yang menjadi kelompok disakarida, yaitu sukrosa (gabungan glukosa dan fruktosa), maltosa (gabungan dari dua unit glukosa), dan laktosa (gabungan glukosa dan galaktosa). Polisakarida merupakan karbohidrat yang terdiri dari banyak satuan (lebih dari delapan satuan) monosakarida, contohnya pati, selulosa, pektin, kitin, dll. Karbohidrat pada umumnya memiliki sifat yaitu, senyawa karbohidrat dari tingkat yang lebih tinggi dapat diubah menjadi tingkat yang lebih rendah dengan cara menghidrolisa, gugus hemiasetal (keton maupun aldehid) mempunyai sifat pereduksi, dan gugus-gugus hidroksil pada karbohidrat juga bertabiat serupa dengan yang terdapat pada gugus alkohol lain. 2.2 Pati Pati merupakan homopolimer glukosa dengan ikatan α-glikosidik yang terdiri dari dua fraksi. Fraksi terlarut disebut amilosa dan fraksi tidak terlarut disebut amilopektin. Rumus umum dari senyawa pati adalah (C 6H10O5)n. Pati atau amilum adalah karbohidrat kompleks (polisakarida) yang bersifat tidak dapat larut dalam air pada temperatur ruangan, berwujud bubuk putih, tawar, tidak berbau, dan dalam bentuk aslinya pati secara alami berbentuk butiranbutiran kecil yang disebut granula. Sebagian besar pati disimpan dalam umbi (ubi kayu, ubi jalar, kentang, dan lain-lain), biji (padi, jagung, gandum, sorghum), batang (sagu), dan buah. Pati dapat dibagi menjadi 2 jenis yaitu pati alami (Native Starch) dan pati yang termodifikasi (Modified Starch). 3 Secara garis besar pati dapat dibedakan atas : a. Amilosa (± 30%) Gambar 2.1 Struktur amilosa Yang mempunyai sifat larut dalam air panas. Merupakan polimer linier dengan ikatan 1,4’ α – D glukosa. Tiap molekul amilosa terdapat ± 250 satuan glukosa. Hidrolisis parsial menghasilkan maltosa dan oligomer lain (maltodextrin) Hidrolisis lengkap hanya menghasilkan D-glukosa. Molekul amilosa membentuk spiral di sekitar molekul I 2 dan antaraksi keduanya akan menimbulkan warna biru. Hal ini digunakan sebagai dasar uji Iod pada pati. b. Amilopektin (± 70%) Gambar 2.2 Struktur amilopektin Mempunyai sifat tidak larut dalam air. Struktur bangun dari senyawa amilopektin hampir sama dengan amilosa, perbedaannya rantai amilopektin mempunyai percabangan. Rantai utama amilopektin mengandung 1,4’–α–D-glukosa, dan percabangan rantai mengandung 1,6’–α – D-glukosa. Tiap molekul mengandung ± 1000 satuan glukosa. 4 Hidrolisa parsial dari amilopektin dapat menghasilkan oligosakarida yang disebut dekstrin, yang sering digunakan sebagai perekat (lem), pasta, dan kanji tekstil. Hidrolisa lanjut dari dekstrin dapat menghasilkan maltosa dan isomaltosa. Hidrolisa lengkap amilopektin hanya menghasilkan D-glukosa. Pati dan juga produk turunannya merupakan bahan yang multiguna dan banyak digunakan pada berbagai industri antara lain pada minuman, makanan yang diproses, kertas, makanan ternak, farmasi dan bahan kimia serta industri nonpangan seperti tekstil, detergent, kemasan dan sebagainya. Dalam industri makanan dapat digunakan sebagai pembentuk gel dan encapsulating agent. Dalam industri kertas dapat digunakan sebagai zat aditive seperti wet-end untuk surface size dan coating binder, bahan perekat. Dapat juga digunakan untuk proses glass fiber sizing. 2.3 Hidrolisa Pati Hidrolisis adalah proses dekomposisi kimia dengan menggunakan air untuk memisahkan ikatan kimia dari substansinya. Hidrolisis pati merupakan proses pemecahan molekul amilum menjadi bagian-bagian penyusunnya yang lebih sederhana seperti dekstrin, isomaltosa, maltosa dan glukosa. Reaksi Hidrolisa pati berlangsung menurut reaksi berikut : (C6H10O5)n + nH2O Pati n(C6H12O6) Glukosa (Yuniwati, 2011) Reaksi antara pati dengan air berlangsung sangat lambat, sehingga perlu bantuan katalisator. Katalisator yang digunakan adalah asam (contoh : HCl, HNO3, H2SO4) dan enzim. Katalisator yang sering digunakan adalah katalisator asam. Asam khlorida (HCl) merupakan asam yang paling sering digunakan sebagai katalis terutama untuk industri makanan karena sifatnya mudah menguap sehingga memudahkan pemisahan dari produknya. Selain itu asam tersebut dapat menghasilkan produk yang berwarna terang. Penggunaan HCl sebagai katalis karena harganya murah, mudah diperoleh dan memiliki efektifitas yang tinggi dalam meningkatkan kecepatan reaksi dan garam yang terbentuk tidak berbahaya, yaitu garam dapur NaCl. 5 2.4 Faktor-Faktor yang Mempengaruhi Hidrolisa Hidrolisa merupakan proses reaktan dengan air untuk memecah senyawa (Coney, 1979 dalam Budiyati dan Bandi, 2015). Faktor-faktor yang mempengaruhi hidrolisa : 1. Katalis Katalis digunakan dalam reaksi hidrolisa untuk mempercepat reaksi. Katalis yang digunakan yaitu enzim atau asam. Katalis asam yang sering digunakan adalah asam klorida (Agra et al., 1973 dalam Budiyati dan Bandi, 2015), asam sulfat, dan asam nitrat. Konsentrasi ion H+ memberikan pengaruh yang besar terhadap laju reaksi dibandingkan dengan jenis asam yang digunakan. Pada umumnya industri banyak menggunakan asam klorida sebagai katalis. Hal ini dikarenakan garam yang terbentuk dalam reaksi netralisasi aman dan dapat dikendalikan dengan air (Budiyati dan Bandi, 2015). 2. Suhu dan Tekanan Pengaruh suhu pada laju reaksi mengikuti persamaan Arrhenius. Pada saat suhu tinggi, laju reaksi juga akan meningkat. Namun ketika suhu reaksi hampir mencapai 0 dan reaksi berada pada fase cair, maka suhu dan temperatur tidak terlalu mempengaruhi keseimbangan (Budiyati dan Bandi, 2015). 3. Pengadukan Laju reaksi akan lebih cepat jika reaktan dapat bertabrakan antara satu dengan yang lain sebaik mungkin. Oleh karena itu, pengadukan sangat diperlukan. Di dalam batch process, hal ini dapat dicapai dengan menggunakan stirrer atau shaker (Agra et al., 1973 dalam Budiyati dan Bandi, 2015). Jika proses merupakan flow process (continuous), maka pengadukan dapat diselesaikan dengan mengatur aliran di dalam reaktor untuk meningkatkan turbulensi (Budiyati dan Bandi, 2015). 4. Perbandingan Reagen Perbandingan reaktan di dalam proses hidrolisa sangat penting. Apabila salah satu reaktan terlalu banyak, maka kesetimbangan akan bergeser ke kanan. Oleh karena itu, suspensi pati dengan kadar yang rendah dapat memberikan hasil yang lebih baik daripada kadar pati yang tinggi. Hal ini dikarenakan molekul pada kadar pati yang lebih tinggi akan sulit bergerak dibandingkan dengan molekul pada kadar pati yang rendah (Budiyati dan Bandi, 2015). 6 2.5 Aplikasi Pati di Bidang Industri 1. Bidang Biofuel Etanol yang dihasilkan dari fermentasi pati hidrolisa dianggap setara dengan alkohol biji-bijian dan dapat digunakan dalam minuman. Hal ini juga memenuhi syarat bebas apabila dicampur dengan bensin pada tingkat 10% sebagai bahan bakar motor. Etanol merupakan komoditas terbarukan yang bisa diproduksi dari bahan-bahan alami. Etanol yang berasal dari bahan alami menawarkan manfaat yang lebih besar jika dibandingkan dengan produkproduk yang berbasis minyak bumi. Ada dua proses dasar untuk memproduksi etanol dari pati yaitu dengan penggilingan basah dan penggilingan kering. Pada awalnya, penggilingan basah merupakan cara yang disukai untuk memproduksi etanol dikarenakan hasil samping dari proses tersebut memiliki harga yang tinggi daripada etanol itu sendiri. Namun karena harga etanol yang kian meningkat, proses penggilingan kering menjadi lebih menguntungkan sebab modal yang dibutuhkan lebih sedikit dibandingkan dengan penggilingan basah (Echkoff dan Watson, 2009). 2. Bidang Industri Kertas Pati merupakan komponen penting dari kualitas kertas. Penggunaan pati di dalam proses pembuatan kertas dan proses konversi kertas menempati urutan ketiga setelah serat selulosa dan pigmen mineral. Pati digunakan sebagai bahan flokulan dan retensi, bahan pengikat, surfice size, pengikat untuk pelapis, dan juga sebagai perekat pada papan. Dispersi pati telah digunakan secara luas dalam pembuatan kertas dan konversi kertas karena sifat uniknya, yaitu merupakan perekat yang terbarukan, murah, viskositas dapat dikontrol, karakteristik reologi yang spesifik, tahan air, memiliki muatan elektrostatik, dapat membentuk film dan ikatan setelah pengeringan (Maurer, 2009). 3. Bidang Plastik Dalam beberapa tahun terakhir ini, pati dimanfaatkan dalam pembuatan plastik yang dapat terurai. Pada awalnya, pati hanya difokuskan pada penggunaan butirannya sebagai pengisi. Dalam perkembangannya, lahirlah termoplastik yang merupakan campuran dari molekul pati dengan polimer vinil hidrofilik, seperti poly(ethylene-co-acrylic acid), poly(ethylene-co-vinyl alcohol), poly(ethylene glycol), polylactic acid, dan polycaprolactone. Selain itu, busa yang kaku serta fleksibel, film, dan bahan bantalan yang mengandung pati juga telah dikembangkan (Maningat et al., 2009). 7 4. Bidang Kosmetik Pati dalam wujud tepung dapat digunakan di dalam bidang kosmetik karena tidak beracun, tidak menyebabkan iritasi, dan tidak menyebabkan sensitivitas. Pati secara umum digunakan sebagai bahan di dalam bubuk kosmetik. Hal ini dikarenakan ukuran pati yang kecil serta luas permukaan, mobilitas, sifat selip, dan daya serap yang besar. Pati dapat meningkatkan kelembutan dan kehalusan produk kosmetik untuk wajah dan tubuh. Selain itu, pati yang telah dimodifikasi telah berhasil diformulasikan dengan krim, lotion, make-up cair, dan bedak kosmetik (Maningat et al., 2009). 8 BAB III METODE PRAKTIKUM 3.1 Alat dan Bahan yang Digunakan 3.1.1 Bahan 1. Tepung singkong 50 gram 2. HCl 37% ; 0,3 N ; ρ = 1,19 gr/ml 3. NaOH 0,5 N ; 20 ml 4. Aquadest secukupnya 5. Glukosa anhidris 0,0025 gr/ml, 250 ml 6. Metilen blue 7. Fehling A 10 ml 8. Fehling B 10 ml 3.1.2 Alat 9. Timbangan 10. Buret 11. Magnetic stirrer plus heater 12. Waterbath 13. Labu leher tiga 14. Thermometer 15. Pendingin balik 16. Klem 17. Statif 18. Pipet volume 19. Pipet tetes 20. Gelas ukur 21. Oven 22. Kompor listrik 23. Erlenmeyer 24. Beaker glass 25. Cawan porselin 26. Corong 27. Indikator pH 9 3.1 Gambar Rangkaian Alat 6 5 7 4 3 2 1 Gambar 3.1 Rangkaian alat Keterangan : 3.2 1. Magnetic stirrer plus heater 2. Waterbath 3. Labu leher tiga 4. Thermometer 5. Pendingin balik 6. Klem 7. Statif Prosedur Praktikum 1. Analisa Kadar Pati a. Persiapan Bahan Tumbuk dan haluskan singkong padat. Hilangkan kadar airnya menggunakan oven sampai berat sampel menjadi konstan. Memasukkan 1 gr tepung singkong tersebut kedalam gelas ukur kemudian tambahkan aquadest 5 ml lalu amati perubahan volume yang terukur. Hitung densitasnya. Hitung massa tepung singkong yang dibutuhkan untuk hidrolisa. b. Standarisasi Larutan Fehling Larutan fehling A sebanyak 5 ml dan larutan fehling B 5 ml dicampur dalam erlenmeyer, lalu ditambah 15 ml larutan glukosa standart dari buret. Campuran dipanaskan hingga 70ºC. Tambahkan 3 tetes indikator metilen blue (MB). Larutan dititrasi dengan glukosa standar hingga warna berubah menjadi merah bata. Catat volume titran (F) yang 10 diperlukan. Proses titrasi dilakukan dalam keadaan panas (diatas kompor), suhu dijaga konstan 65ºC - 70ºC. c. Penentuan kadar pati Sebanyak 16,205 gram tepung singkong, 4,947 ml katalis HCl, dan 180,387 ml aquadest dimasukkan ke dalam labu leher tiga dan dipanaskan hingga suhu 70ºC selama 1,5 jam dengan disertai pengadukan. Setelah waktu operasi selesai, campuran kemudian didinginkan, diencerkan dengan aquades sampai 500 ml, dan dinetralkan menggunakan NaOH. Kemudian campuran yang sudah netral diambil sebanyak 5 ml dan diencerkan sampai 100 ml. Campuran yang sudah diencerkan kemudian diambil sebanyak 5 ml dan ditambahkan 5 ml fehling A, 5 ml fehling B, 15 ml glukosa standar lalu dipanaskan sampai 70ºC. Kemudian tambahkan 3 tetes indikator MB. Larutan dititrasi dengan glukosa standar hingga warna berubah menjadi merah bata. Catat kebutuhan titran (M). Hitung kadar pati. Yang perlu diperhatikan, proses titrasi dilakukan dalam keadaan panas (di atas kompor) dengan suhu dijaga konstan 60ºC - 70ºC. B = 500 ml Jika ingin diperoleh kadar pati, nilai X dikalikan dengan 0,9. Keterangan : X = hasil glukosa, dalam bagian berat pati. F = larutan glukosa standart yang diperlukan, ml. M = larutan glukose standart yang digunakan untuk menitrasi sampel, ml. 2. N = gr glukose / ml larutan standart = 0,0025 gr/ml. W = berat pati yang dihidrolisis, gram. B = volume pengenceran suspensi pati. Pembuatan larutan fehling a. Larutan Fehling A Dibuat dengan melarutkan 34,639 gram CuSO4.5H2O dalam 500 ml aquades. Zat padat yang tidak larut disaring. 11 b. Larutan Fehling B Dibuat dengan malarutkan 172 gram Kalium Natrium Tartrat (KNaC4H4O6.4H2O) dan 50 gram NaOH dalam aquades sampai volumenya menjadi 500 ml lalu dibiarkan selama 2 hari. Selanjutnya larutan disaring dengan wol glass. 3. Pembuatan Larutan Glukosa standart Dibuat dengan melarutkan 0,625 gram glukosa anhidris dengan air suling sampai volume 250 ml. 12 BAB IV HASIL DAN PEMBAHASAN 4.1 Perbandingan Kadar Praktis dan Teoritis Bedasarkan praktikum karbohidrat untuk menganalisa kadar pati dalam tepung singkong dengan metode hidrolisis menggunakan katalis asam (HCl) diperoleh data yang disajikan dalam tabel berikut. Tabel 4.1 Perbandingan kadar pati tepung singkong acuan dengan kadar pati tepung singkong praktis Sampel Kadar Pati Praktis Tepung Singkong 81,45% Kadar Pati Acuan 85,045% (Novitasari dan Arief, 2018) Kadar pati tepung singkong praktis lebih kecil daripada kadar pati acuan disebabkan oleh adanya sifat fisikokimia pati. Sifat fisikokimia pati sangat dipengaruhi oleh kondisi lingkungan selama pertumbuhan tanaman, terutama pertumbuhan akar. Sifat ini juga terkait dengan kecenderungan perilaku pati properti fungsional seperti suhu tinggi dan viskositas rendah. Pati yang ditanam di daerah dengan suhu yang lebih hangat akan menghasilkan butiran yang lebih kecil, kandungan amilosa yang tinggi, serta suhu dan entalpi yang lebih tinggi, begitu juga dengan sebaliknya. Suhu yang lebih tinggi berbanding lurus dengan viskositas yang rendah. Viskositas yang rendah ini dapat dikaitkan dengan lebih rendahnya kadar pati di dalam tepung singkong (Aldana dan Quintero, 2013). Dengan suhu yang tinggi, maka pati akan dapat lebih mudah larut dalam proses hidrolisa. Kelarutan pati ini dikarenakan pemendekan panjang rantai pati, yang mana juga diikuti melemahnya ikatan hidrogen (Osunsami dkk., 1989 dalam Omojola dkk., 2011) atau karena peningkatan gugus hidroksil (Aiyeleye dkk., 1983 dalam Omojola dkk., 2011). Kelarutan pati yang tinggi ini juga dapat disebabkan oleh hilangnya struktur butiran dan pelepasan amilosa dari butiranbutiran pati. Amilosa yang memisahkan diri dari butiran pati inilah yang ikut serta di dalam meningkatnya larutan (Marcon dkk., 2007 dalam Thys dkk., 2013). Oleh karena itu, kadar pati yang didapatkan dari proses hidrolisa ini rendah. Apabila konsentrasi asam di dalam larutan pada saat hidrolisa terlalu berlebihan, maka akan menyebabkan waktu yang dibutuhkan untuk hidrolisis semakin singkat karena pati lebih cepat terhidrolisis. Kecenderungan penurunan kandungan pati oleh asam yang mana akan menipiskan pati seiring dengan bertambahnya waktu reaksi (Babu dkk., 2015). Asam akan menyerang daerah amorf dan kristal dari butiran pati untuk mendapatkan molekul air. Asam akan 13 menghidrolisis daerah amorf dari molekul pati dan menghasilkan pengurangan yang signifikan dalam rantai amilosa yang panjang dan mengakibatkan pelarutannya mengalami peningkatan. Hilangnya amilosa ini juga secara tidak langsung akan menurunkan titik leleh butiran pati (Thys dkk., 2013). Semakin rendah suhu yang dibutuhkan, maka waktu yang dibutuhkan juga semakin singkat. Hal-hal inilah yang mempengaruhi rendahnya kadar pati. 4.2 Mekanisme Hidrolisa Pati dengan Katalis Asam Hidrolisa pati dapat dilakukan dengan menggunakan asam atau enzim. Hidrolisis asam ditemukan pada awal abad ke-19 ketika seorang ahli kimia Jerman, Kirchoff menunjukkan bahwa merebus pati gandum dengan asam sulfat encer, dapat diperoleh sirup manis. Kemudian, pati kentang digunakan sebagai sumber pati dan asam sulfat diganti dengan asam klorida dan pemanasan tidak langsung dari bejana reaksi biasa terjadi. Sejak itu, asam telah banyak digunakan untuk pemecahan pati menjadi glukosa (Dziedzic dan Kearsley, 2012 dalam Azmi dkk., 2017). Dalam hidrolisis asam, ion hidroksonium (H3O+) melakukan serangan elektrofilik pada atom oksigen dari ikatan glikosidik α (1 → 4) (Gambar 4.1a). Pada langkah selanjutnya, elektron di salah satu ikatan karbon-oksigen bergerak ke atom oksigen (Gambar 4.1b) untuk menghasilkan zat antara karbokation berenergi tinggi yang tidak stabil (Gambar 4.1c). Antara karbokation intermediet adalah asam Lewis, sehingga selanjutnya bereaksi dengan air (Gambar 4.1d), basa Lewis mengarah ke regenerasi gugus hidroksil (Gambar 4.1e) (Hoover, 2000). Reaksi hidrolisa pati dengan katalis asam adalah sebagai berikut : hidrolisa (C6H10O5)n + nH2O → nC6H12O6 14 Gambar 4.1 Mekanisme Hidrolisis Pati dengan Katalis Asam (Hoover, 2000) Hidrolisa asam telah digunakan untuk memodifikasi struktur butiran pati dan menghasilkan larutan pati. Penggunaan hidrolisis pati oleh asam pada industri adalah sebagai pra modifikasi langkah produksi pati kationik dan amfoter, sebagai bahan pengatur ukuran lungsin untuk meningkatkan kekuatan benang dan ketahanan abrasi dalam operasi penenunan, untuk persiapan permen karet pati, untuk pembuatan papan gipsum sebagai konstruksi dinding kering, dan untuk pembuatan kertas dan karton (Hoover, 2000). Amilodekstrin beras dibuat dengan menghidrolisis pati beras dalam larutan asam (4% HCl) alkohol (70%) pada suhu 78-80° C mudah dilarutkan dengan air hangat (50° C). Emulsi dibuat dengan mengganti sebagian dari minyak (digunakan dalam formulasi emulsi jenis mayonaise) dengan amilodekstrin beras, menunjukkan viskositas dan stabilitas tinggi. Hal ini membuat amilodekstrin dapat dijadikan sebagai pengganti lemak (Chun et al., 1997 dalam Hoover, 2000). 4.3 Mekanisme Penentuan Kadar Pati dengan Uji Fehling Uji Fehling digunakan secara luas dalam uji karbohidrat. Reagen Fehling biasanya digunakan untuk gula pereduksi tetapi diketahui tidak spesifik untuk aldehida. Hasil uji Fehling pada karbohidrat ditunjukkan dengan terbentuknya endapan berwarna merah bata. Larutan Fehling mengandung larutan hidroksida cupric alkali biru, yang dipanaskan dengan gula pereduksi direduksi menjadi 15 cuprous oksida kuning atau merah dan diendapkan. Oleh karena itu, pembentukan endapan berwarna kuning atau merah kecoklatan membantu dalam mendeteksi gula pereduksi dalam larutan uji (Mohamed, 2019). Reaksi penentuan kadar pati dengan uji Fehling : R-CHO + Cu++ → Cu+ + OH- → R-COOH + Cu+ ∆ CuOH → Cu2O W.B Red ppt (Mohamed, 2019) Uji Fehling memanfaatkan reaktivitas siap aldehida dengan menggunakan Red ppt ion cupri zat pengoksidasi lemah (Cu2+) dalam larutan∆ basa. Selain ion tembaga, larutan Fehling mengandung ion tartrat sebagai W.B agen pengompleks untuk menjaga ion tembaga tetap dalam larutan. Tanpa ion tartrat, cupric hidroksida akan mengendap dari larutan basa. Ion tartrat tidak dapat membentuk ion tembaga kompleks Cu+, sehingga reduksi Cu2+ menjadi Cu+ dengan gula reduksi menghasilkan endapan Cu2O berwarna oranye menjadi merah (Mohamed, 2019). 16 BAB V PENUTUP 5.1 Kesimpulan 1. Berdasarkan percobaan ini, diperoleh kadar pati tepung singkong praktis sebesar 81,45%. Sedangkan, kadar pati tepung singkong acuan dari jurnal sebesar 85,045%. Kadar pati praktis lebih kecil daripada kadar pati acuan hal ini disebabkan oleh beberapa faktor. Faktor-faktor tersebut adalah adanya sifat fisikokimia pati, kelarutan, dan konsentrasi asam di dalam larutan pada saat hidrolisa terlalu berlebihan. 2. Mekanisme hidrolisis asam adalah ion hidroksonium melakukan serangan elektrofilik pada atom oksigen. Selanjutnya, elektron di salah satu ikatan karbon-oksigen bergerak ke atom oksigen. Terdapat asam Lewis di antara karbokation intermediet yang selanjutnya bereaksi dengan air dan basa Lewis mengarah ke regenerasi gugus hidroksil. Aplikasi hidrolisis pati oleh asam pada industri adalah meningkatkan kekuatan benang dan ketahanan abrasi pada penenunan, persiapan permen karet pati, dan pembuatan papan gipsum. 3. Salah satu uji karbohidrat yang sering digunakan adalah uji Fehling menggunakan reagen Fehling yang mengandung larutan hidroksida cupric alkali biru. Selain itu, reagen Fehling juga mengandung ion tartrat yang berperan sebagai agen pengompleks untuk menjaga agar ion tembaga tetap berada dalam larutan. Adanya karbohidrat ditunjukkan dengan terbentuknya endapan merah bata. Warna merah bata ini ditimbulkan dari reduksi Cu 2+ menjadi Cu+. 5.2 Saran 1. Larutan Fehling dijaga agar tidak terkontaminasi dan HCl dijada agar tidak menguap. 2. Kecepatan magnetic stirrer diperhatikan agar tidak menimbulkan pusaran (vortex). 3. Titrasi harus dilakukan di atas kompor listrik untuk menjaga suhu larutan yang dititrasi agar konstan. 17 DAFTAR PUSTAKA Aldana, A. S. dan Quintero, A. F. 2013. Physicochemical characterization of two cassava (Manihot esculenta Crantiz). Scientia Agroalimentaria, Vol. 1, 1925. A.O.A.C., Oficial Method of Analysis of the A.O.A.C., 11 ed, p.539 – 540, Washington, D.C., 1970. Azmi, A.S., Malek, M. I. A., dan Puad, N. I. M. 2017. A review on acid and enzymatic hydrolyses of sagp starch. International Food Research Journal, 24(Suppl), 265-273. Babu, A. S., Parimalavalli, R., dan Rudra, S. G. 2015. Effect of citric acid concentration and hydrolysis time on physicochemical properties of sweet potato starches. International Journal of Biological Macromolecules, 80, 557565. Budiyati, E. dan Bandi, U. 2015. The Effect of Hydrolysis Temperature and Catalyst Concentration on Bio-ethanol Production from Banana Weevil. Proceedings of The 9th Joint Conference on Chemistry. Semarang : Chemistry Department, FSM, Diponegoro University. Echkoff, S. R. dan Watson, S. A. 2009. Corn ad sorghum starches : production. Starch : Chemistry and Technology, 3(9), 374-431. Groggins, PH, Unit Processes in Organic Synthesis, 5 ed, pp. 750 – 783, Mc Graw HillBook Company Inc, New York, 1950. Herawati, Heny. 2010. Potensi Pengembangan Produk Pati Tahan Cerna Sebagai Pangan Fungsional. Ungaran: Balai Pengkajian Teknologi Pertanian Jawa Tengah. Hoover, R. 2000. Acid-treated starches. Food Reviews International, 16(3), 369-392. Kerr, R. W., “Chemistry and Industry of Starch”, 2 ed, pp. 375 – 403, Academic Press, Inc, New York, 1950. Maningat, C. D., Seib, P. A., Bassi, S. D., Woo, K. S., dan Lasater, G. D. 2009. Wheat starch : Production, properties, modification, and uses. Starch : Chemistry and Technology, 3(10), 442-491. Maurer, H. W. 2009. Starch in the paper industry. Starch : Chemistry and Technology, 3(18), 658-706. Mohamed, A. M. H. 2019. Course Book of Chemistry 2 (Biochemistry). Benha : Department of Biochemistry, Benha University. 18 Novitasari, Erliana dan Arief, Ratna Wylis. 2018. Analisis Karakteristik Kimia Tepung Kasava dari Ubikayu Varietas Klenteng dan Casessart (UJ5). Jurnal Penelitian Pertanian Terapan, 18(1), 52-58. Omojola, M. O., Manu, N., dan Thomas, S. A. 2011. Effect of acid hydrolysis on the physicochemical properties of cola starch. African Journal of Pure and Applied Chemistry, 5(9), 307-315. Robyt, John F., “Essential of Carbohydrate Chemistry”. Springer, New York, NY, 1998. Thys, R. C. S., Aires, A. G., Marczak, L. D. F., dan Norena, C. P. Z. 2013. The effect of acid hydrolysis on the technological functional properties of pinhao (Araucaria brasiliensis) starch. Ciencia e Tecnologia de Alimentos, 33(1), 89-94. Woodman, A., “Food Analysis”, 4ed, pp. 264 – 265, Mc Graw Hill Book Company, Inc, New York, 1941. Yuniwati, M., Dian Ismiyati, dan Reny Kurniasih. 2011. Kinetika Reaksi Hidrolisis Pati Pisang Tanduk dengan Katalisator Asam Chlorida. Jurnal Teknologi Vol. 4, No. 2. 19 LAPORAN SEMENTARA PRAKTIKUM DASAR TEKNIK KIMIA II Materi : Karbohidrat GROUP : 7 - Senin REKAN KERJA : 1. Aurellia Livia Hidayat NIM. 21030120130110 2. Desita Rachmawanti NIM. 21030120120011 3. Ergian Janitra NIM. 21030120130103 4. Ghea Fsyifa Hidawati NIM. 21030120120025 LABORATORIUM DASAR TEKNIK KIMIA DEPARTEMEN TEKNIK KIMIA FAKULTAS TEKNIK UNIVERSITAS DIPONEGORO SEMARANG 2021 A-1 I. TUJUAN PERCOBAAN 1. Menyusun rangkaian alat analisa karbohidrat dan mengoperasikannya. 2. Memahami reaksi-reaksi pada uji penentuan kadar pati. 3. Menentukan kadar karbohidrat (pati) pada suatu tepung singkong dengan prosedur yang benar II. PERCOBAAN 2.1 2.2 Bahan yang Digunkana 1. Tepung singkong 50 gram 2. HCl 37% ; 0,3 N ; ρ = 1,19 gr/ml 3. NaOH 0,5 N ; 20 ml 4. Aquadest secukupnya 5. Glukosa anhidris 0,0025 gr/ml, 250 ml 6. Metilen blue 7. Fehling A 10 ml 8. Fehling B 10 ml Alat yang Dipakai 1. Timbangan 11. Pipet tetes 2. Buret 12. Gelas ukur 3. Magnetic stirrer plus heater 13. Oven 4. Waterbath 14. Kompor listrik 5. Labu leher tiga 15. Erlenmeyer 6. Thermometer 16. Beaker glass 7. Pendingin balik 17 Cawan porselen 8. Klem 18. Corong 9. Statif 19. Indikator Ph A-2 Rangkaian Alat Keterangan : 6 5 7 4 3 2 1. Magnetic stirrer plus heater 2. Waterbath 3. Labu leher tiga 4. Thermometer 5. Pendingin balik 6. Klem 7. Statif 1 Gambar 2.1 Rangkaian Alat 2.3 Cara Kerja 2.3.1 Analisa Kadar Pati a. Persiapan Bahan Tumbuk dan haluskan singkong padat. Hilangkan kadar airnya menggunakan oven sampai berat tepung singkong menjadi konstan. Memasukkan 1 gr tepung singkong tersebut kedalam gelas ukur kemudian tambahkan aquadest 5 ml lalu amati perubahan volum yang terukur, hitung densitasnya. Hitung massa tepung singkong yang dibutuhkan untuk hidrolisa. b. Standarisasi Larutan Fehling Larutan fehling A sebanyak 5 ml dan larutan fehling B 5 ml dicampur dalam erlenmeyer, lalu ditambah 15 ml larutan glukosa standart dari buret. Campuran dipanaskan hingga 70ºC. Tambahkan 3 tetes indikator metilen blue (MB). Larutan dititrasi dengan glukosa standar hingga warna berubah menjadi merah bata. Catat volume titran (F) yang diperlukan, proses titrasi dilakukan dalam keadaan panas (diatas kompor), suhu dijaga konstan 65ºC-70ºC. c. Penentuan Kadar Pati Sebanyak 16,205 gram tepung singkong, 4,947 ml katalis HCl, dan 180,387 ml aquadest dimasukkan ke dalam labu leher tiga dan dipanaskan hingga suhu 70ºC selama 1,5 jam dengan disertai pengadukan. Setelah waktu operasi selesai, campuran kemudian didinginkan, diencerkan dengan aquades sampai 500 ml, dan dinetralkan menggunakan NaOH. Kemudian campuran yang A-3 sudah netral diambil sebanyak 5 ml dan diencerkan sampai 100 ml. Campuran yang sudah diencerkan kemudian diambil sebanyak 5 ml dan ditambahkan 5 ml fehling A, 5 ml fehling B, 15 ml glukosa standar lalu dipanaskan sampai 70ºC. Kemudian tambahkan 3 tetes indikator MB. Larutan dititrasi dan catat kebutuhan titran (M). Hitung kadar pati. Yang perlu diperhatikan, proses titrasi dilakukan dalam keadaan panas (di atas kompor) suhu dijaga konstan 60ºC - 70ºC. Dengan B = 500 ml, jika ingin diperoleh kadar pati dikalikan dengan 0,9. Keterangan : X = hasil glukosa, dalam bagian berat pati. F = larutan glukosa standart yang diperlukan, ml. M = larutan glukosa standart yang digunakan untuk menitrasi tepung tapioka, ml. N = gr glukosa / ml larutan standart = 0,0025 gr/ml. W = berat pati yang dihidrolisis, gram. B = volume pengenceran suspensi pati. 2.3.2 Pembuatan Larutan Fehling a. Larutan Fehling A Dibuat dengan melarutkan 34,639 gram CuSO4.5H2O dalam 500 ml aquades. Zat padat yang tidak larut disaring. b. Larutan Fehling B Dibuat dengan malarutkan 172 gram Kalium Natrium Tartrat (KNaC4H4O6.4H2O) dan 50 gram NaOH dalam aquadest sampai volumenya menjadi 500 ml lalu dibiarkan selama 2 hari. Selanjutnya larutan disaring dengan wol glass. 2.3.3 Pembuatan Larutan Glukosa Standart Dibuat dengan melarutkan 0,625 gram glukosa anhidris dengan air suling sampai volume 250 ml A-4 2.4 Hasil Percobaan Massa sampel untuk mencari densitas : 0,0996 gram V awal : 5 ml V akhir : 5,9 ml V HCl : 4,974 ml Massa NaOH : 0,4 gram Massa Gluka Anhidris : 0,625 gram V sampel : 14,639 ml ρ sampel : 1,107 gram/ml Massa sampel yang dihidrolisa : 16,205 gram 1. 2. Volume Glukosa Standar yang Diperlukan (F) F1 (ml) F2 (ml) F3 (ml) Rata-Rata (ml) 9,5 9,2 9,1 9,267 Volume Larutan Glukosa Standar untuk Menitrasi Tepung Singkong (M) M1 (ml) M2 (ml) M3 (ml) Rata-Rata (ml) 6,3 6,2 6,5 6,333 MENGETAHUI PRAKTIKAN Aurellia Livia Hidayat Desita Rachmawanti NIM. 20130120130110 NIM. 21030120120011 Ergian Janitra ASISTEN Vincent Hartanto NIM. 21030118130144 Ghea Fsyifa Hidawati NIM. 21030120130103 NIM. 21030120120025 A-5 LEMBAR PERHITUNGAN 1. Titrasi Standarisasi Larutan Fehling (F) Diketahui : F1 = 9,5 ml F2 = 9,2 ml F3 = 9,1 ml Ditanya : F̅ Jawab : F̅ = =…? 𝐹1+𝐹2+𝐹3 3 F̅ = 9,5 𝑚𝑙+9,2 𝑚𝑙+9,1 𝑚𝑙 F̅ = 27,8 𝑚𝑙 3 3 F̅ = 9,267 𝑚𝑙 2. Titrasi Penentuan Kadar Pati Diketahui : M1 = 6,3 ml M2 = 6,2 ml M3 = 6,5 ml Ditanya : M̅ =…? Jawab ̅ = :M 𝑀1+𝑀2+𝑀3 3 M̅ = 6,3 𝑚𝑙+6,2 𝑚𝑙+6,2 𝑚𝑙 M̅ = 19 𝑚𝑙 3 3 ̅ = 6,333 𝑚𝑙 M 3. Penentuan Kadar Pati Diketahui : F̅ = 9,267 ml M̅ = 6,333 ml N = 0,0025 gr/ml W = 16,205 gr B = 500 ml Ditanya : % Pati = … ? Jawab :𝑥= ̅ −M ̅ )N(100)(𝐵) (F 5 5 𝑊 B-1 𝑥= 𝑥= (9,267 ml − 6,333 ml)(0,0025 𝑔𝑟⁄𝑚𝑙 )( 100 500 )( ) 5 5 16,205 𝑔𝑟 (2,934 ml)(0,0025 𝑔𝑟⁄𝑚𝑙)(20)(100) 16,205 𝑔𝑟 𝑥 = 0,905 % 𝑃𝑎𝑡𝑖 = 𝑥 × 0,9 × 100% % 𝑃𝑎𝑡𝑖 = 0,905 × 0,9 × 100% % 𝑃𝑎𝑡𝑖 = 81,45% B-2 LEMBAR KUANTITAS REAGEN LABORATORIUM DASAR TEKNIK KIMIA II DEPARTEMEN TEKNIK KIMIA FAKULTAS TEKNIK UNIVERSITAS DIPONEGORO LEMBAR KUANTITAS REAGEN MATERI HARI/TANGGAL KELOMPOK NAMA : : : : ASISTEN : Karbohidrat Senin, 1 Maret 2021 7 Senin 1. Aurellia Livia Hidayat 2. Desita Rachmawanti 3. Ergian Janitra 4. Ghea Fsyifa Hidawati Vincent Hartanto KUANTITAS REAGEN NO. JENIS REAGEN KUANTITAS 1. Tepung Singkong 50 gram 2. HCl 37 % ; 0,3 N ; (⍴ = 1,19 gr/mL) Sesuaikan 3. NaOH 0.5 N 20 Ml 4. Fehling A dan Fehling B @ 5 Ml 5. Indikator Metilen Blue (MB) @ 3 tetes 6. Glukosa Anhidris (⍴ = 0,0025 gr/mL) 250 Ml 7. Aquadest Sesuaikan TUGAS TAMBAHAN : Mencari jurnal kadar pati Tepung Singkong (ACC DATA) Faktor – faktor yang mempengaruhi hidrolisa (Tambahkan di bab 2) Mencari dan mempelajari mekanisme Uji Fehling (ACC DATA) Aplikasi Pati dalam Bidang Industri (Tambahkan di bab 2) Mencari jurnal kadar pati Tepung Singkong (ACC DATA) Faktor – faktor yang mempengaruhi hidrolisa (Tambahkan di bab 2) Mencari dan mempelajari mekanisme Uji Fehling (ACC DATA) CATATAN Aplikasi Pati dalam Bidang Industri (Tambahkan di bab 2)Semarang, 24 Februari 2021 ASISTEN T Hidrolisa = 70˚C t Hidrolisa = 1,5 jam T Titrasi = 65˚C - 70˚C % suspense = 8% Volume basis = 200 mL Vincent Hartanto NIM. 21030118130144 C-1 LEMBAR PERHITUNGAN REAGEN 1. Menghitung Densitas Sampel Massa sampel = 0,996 gram Volume awal = 5 ml Volume akhir = 5,9 ml 𝑀𝑎𝑠𝑠𝑎 𝑠𝑎𝑚𝑝𝑒𝑙 𝑉𝑜𝑙𝑢𝑚𝑒 𝑎𝑘ℎ𝑖𝑟 − 𝑉𝑜𝑙𝑢𝑚𝑒 𝑎𝑤𝑎𝑙 0,996 𝑔𝑟 ρ sampel = 5,9 𝑚𝑙 − 5 𝑚𝑙 ρ sampel = 0,996 𝑔𝑟 0,9 𝑚𝑙 𝑔𝑟 ρ sampel = 1,107 ⁄𝑚𝑙 ρ sampel = 2. Menghitung Volume HCl V basis = 200 ml ρ HCl = 1,19 gr/ml BM HCl = 36,5 N HCl = 0,3 N Kadar HCl = 37% 𝑔𝑟 𝐻𝐶𝑙 1000 × × 𝑣𝑎𝑙𝑒𝑛𝑠𝑖 × 𝑘𝑎𝑑𝑎𝑟 𝐵𝑀 𝐻𝐶𝑙 𝑉 𝑏𝑎𝑠𝑖𝑠 (ρ × V) 𝐻𝐶𝑙 1000 𝑁= × × 𝑣𝑎𝑙𝑒𝑛𝑠𝑖 × 𝑘𝑎𝑑𝑎𝑟 𝐵𝑀 𝐻𝐶𝑙 𝑉 𝑏𝑎𝑠𝑖𝑠 𝑔𝑟 (1,19 ⁄𝑚𝑜𝑙 × 𝑉 𝐻𝐶𝑙) 1000 0,3 𝑁 = × × 1 × 37% 36,5 200 𝑚𝑙 𝑁= 𝑉 𝐻𝐶𝑙 = 4,947 𝑚𝑙 3. 4. Menghitung Volume Sampel V basis = V HCl + V aquadest + V sampel 200 ml = 4,947 ml + V aquadest + V sampel V sampel = 195,026 ml – V aquadest Menghitung Massa Sampel yang Dibutuhkan % suspensi = 8% % suspensi = 𝑚𝑎𝑠𝑠𝑎 𝑠𝑎𝑚𝑝𝑒𝑙 𝑚𝑎𝑠𝑠𝑎 𝑏𝑎𝑠𝑖𝑠 D-1 % suspensi = 𝑚𝑎𝑠𝑠𝑎 𝑠𝑎𝑚𝑝𝑒𝑙 𝑚𝑎𝑠𝑠𝑎 𝐻𝐶𝑙 + 𝑚𝑎𝑠𝑠𝑎 𝑎𝑞𝑢𝑎𝑑𝑒𝑠𝑡 + 𝑚𝑎𝑠𝑠𝑎 𝑠𝑎𝑚𝑝𝑒𝑙 (ρ × V) 𝑠𝑎𝑚𝑝𝑒𝑙 (ρ × V) 𝐻𝐶𝑙 + (ρ × V) aquadest + (ρ × V) 𝑠𝑎𝑚𝑝𝑒𝑙 𝑔𝑟 1,107 ⁄𝑚𝑙 × (195,026 𝑚𝑙 − 𝑉 𝑎𝑞𝑢𝑎𝑑𝑒𝑠𝑡) 0,08 = 𝑔𝑟 𝑔𝑟 (1,19 ⁄𝑚𝑙 × 4,947 𝑚𝑙) + (1 ⁄𝑚𝑙 × V aquadest) 𝑔𝑟 + (1,107 ⁄𝑚𝑙 × (195,026 ml − V aquadest)) % suspensi = 0,08 = 215,894 − 1,107 𝑉 𝑎𝑞𝑢𝑎𝑑𝑒𝑠𝑡 5,919 + 𝑉 𝑎𝑞𝑢𝑎𝑑𝑒𝑠𝑡 + 215,894 − 1,107 𝑉 𝑎𝑞𝑢𝑎𝑑𝑒𝑠𝑡 0,08 = 215,894 − 1,107 𝑉 𝑎𝑞𝑢𝑎𝑑𝑒𝑠𝑡 221,813 − 0,107 𝑉 𝑎𝑞𝑢𝑎𝑑𝑒𝑠𝑡 V aquadest = 180,3871 ml V sampel = 195,026 ml – V aquadest = 195,026 ml – 180,3871 ml = 14,6398 ml = 14,639 ml 5. Menghitung Massa NaOH N NaOH = 0,5 N V basis NaOH = 20 ml BM NaOH = 40 𝑔𝑟 𝑁𝑎𝑂𝐻 1000 × × 𝑣𝑎𝑙𝑒𝑛𝑠𝑖 𝐵𝑀 𝑁𝑎𝑂𝐻 𝑉 𝑏𝑎𝑠𝑖𝑠 𝑁𝑎𝑂𝐻 𝑔𝑟 𝑁𝑎𝑂𝐻 1000 0,5 𝑁 = × ×1 40 20 𝑚𝑙 𝑁= 𝑚𝑎𝑠𝑠𝑎 𝑁𝑎𝑂𝐻 = 0,4 𝑔𝑟𝑎𝑚 6. Menghitung Massa Glukosa Anhidris ρ = 0,0025 gr/ml V basis = 250 ml Massa = ρ × V basis = 0,0025 gr/ml × 250 ml = 0,625 gram D-2 REFERENSI E-1 9 Corn and Sorghum Starches: Production Steven R. Eckhoff 1 and Stanley A. Watson2 1 Department of Agricultural Engineering University of Illinois, Urbana, Illinois, USA 2 Ohio Agricultural Research and Development Center The Ohio State University, Wooster, Ohio, USA (Retired) I. Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . II. Structure, Composition and Quality of Grain . . . . . . . . . . . . . . . . . . . . . . 1. Structure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2. Composition . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3. Grain Quality . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . III. Wet-milling . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1. Grain Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2. Steeping . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3. Milling and Fraction Separation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4. Starch Processing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5. Product Drying, Energy Use and Pollution Control . . . . . . . . . . . . . . . 6. Automation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . IV. The Products . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1. Starch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2. Sweeteners . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3. Ethanol. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4. Corn Oil . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5. Feed Products. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . V. Alternative Fractionation Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . VI. Future Directions in Starch Manufacturing . . . . . . . . . . . . . . . . . . . . . . . . 1. Continued Expansion into Fermentation Products . . . . . . . . . . . . . . . . 2. Biosolids as Animal Food . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3. Processing of Specific Hybrids . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4. New Corn Genotypes and Phenotypes via Biotechnology and Genetic Engineering . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5. Segregation of the Corn Starch Industry. . . . . . . . . . . . . . . . . . . . . . . . VII. References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Starch: Chemistry and Technology, Third Edition ISBN: 978-0-12-746275-2 374 375 376 381 385 391 392 394 408 421 421 423 423 423 423 424 425 426 427 429 429 429 430 430 430 431 Copyright © 2009, Elsevier Inc. All rights reserved E-2 424 Corn and Sorghum Starches: Production Table 9.10 Analysis and properties of powdered corn and sorghum starchesa Corn Starch (%) Moisture (%) Protein (N 6.25) (%) Ash (%) Fat (by ether extraction) (%) Lipids, total (%)b SO2 (mg/kg)c Crude fiber (%) pH Linear starch fraction (amylose) (%) Branched starch fraction (amulopectin) (%)d Granule size (microns)e Average granule size (microns)e Granule gelatinization temperature range (°C)f Swelling power at 95°Cg Solubility at 95°Cg Specific gravity Weight per cubic foot (pounds) Sorghum Waxy Normal Normal 88 11 0.28 0.1 0.04 0.23 – 0.1 5 0 100 – – 63–72 64 23 1.5 44–45 88 11 0.35 0.1 0.04 0.87 49 0.1 5 28 72 5–30 9.2 62–72 24 25 1.5 44–45 88 11 0.37 0.1 0.06 0.72 – 0.2 5 28 72 4–25 15 68–75 22 22 1.5 44–45 a Reference 233, except as noted. Values for waxy corn and sorghum from unpublished data except as noted b Reference 235 c Reference 237 d Percentage of carbohydrate e Reference 236 f Initial and end temperatures for loss of microscopic birefringence234,236 g Reference 234 properties of the most popular of the liquid sweetener products.238 Three other products are normally sold in dry form: D-glucose (dextrose) in the monohydrate and anhydrous crystal forms; very low DE (22–30) corn syrup; and maltodextrins (5–20 DE). The latter two products are sold as amorphous powders. 3. Ethanol Ethanol produced by fermentation of starch hydrolyzates is regarded legally as equivalent to grain alcohol and may be used in beverages. It also qualifies for taxexempt status when blended with gasoline at a level of 10% for use as a motor fuel. Ethanol is a renewable commodity when produced from a biological material, has a current net energy ratio (energy from ethanol:energy to produce corn and ethanol) of 2.51:1,239 and offers societal benefits when compared to petroleum-based products. Ethanol production in the US increased dramatically in a three-year period around 2005, to the point that use of corn for ethanol production became almost twice that used for starch production. E-3 IV. The Products 425 Table 9.11 Properties of commercial corn syrups234 Acid Conversion, DE level Acid-enzyme 43° 80 20 43° 80.3 19.7 43° 81 19 43° 80.3 19.7 43° 82 18 43° 82.2 17.8 – 71 29 – 71 29 37 0.4 42 0.4 52 0.4 42 0.4 62 0.4 69 0.4 96 0.03 (95) 0.03 Monosaccharides (%) D-Glucose (%) Fructose (%) Disaccharides (%) Trisaccharides (%) Tetrasaccharides (%) Pentasaccharides Hexasaccharides (%) Higher saccharides (%) 15 0 12 11 10 8 6 38 19 0 14 12 10 8 6 31 28 0 17 13 10 8 6 18 6 0 45 15 2 1 1 30 39 0 28 14 4 5 2 8 50 0 27 8 5 3 2 5 93 0 4 52 42 3 46 55 2 3 3 2 Viscosity, centipoises at: 24E 37.7E 44E 15000 30000 8000 56000 14500 4900 31500 8500 2900 56000 14500 4900 22000 6000 2050 – – – – – – – – – – – – Commercial Baume Solids (%) Moisture (%) Dry basis Dextrose equivalent Ash (sulfated) (%) Carbohydrate composition Enzyme–enzyme There are two basic processes for ethanol production. One is traditional wet-milling; the other is the dry grind process, sometimes referred to as dry-milling. Until the early part of the twenty-first century, wet-milling was the preferred means of producing ethanol because the co-products of wet-milling are of greater value than those from the dry grind process. Because ethanol had a low price, the value of the co-products was the difference between profitability and negative revenue. However, as the price of ethanol increased, the dry grind process became more profitable than wet-milling, because of its lower capital requirements and higher yield of ethanol per unit weight of corn. Modified dry grind processes have been proposed and offer to increase the co-product value.240–243 4. Corn Oil About 70 kg of crude corn oil is recovered from the germ isolated from a metric ton of corn (1260 bushels). The crude oil is refined by standard methods to reduce the content of free fatty acids, waxes, phospholipids, color and miscellaneous unsapponifiable substances. Its low solidifying point, low smoke point and slightly ‘corny’ flavor make it a preferred oil for household use, where 50–60% of the production is utilized. Nearly all the remainder is used in the manufacture of oleomargarine. The high level of linoleic acid is claimed to be a dietary advantage. The low level of linolenic acid and an adequate level of tocopherols contribute to corn oil’s good oxidative stability.53 Grain sorghum oil is similar in fatty acid composition to corn oil; the crude oil has a higher wax content and is more difficult to refine. E-4 18 Starch in the Paper Industry Hans W. Maurer Highland, Maryland 20777 I. II. III. IV. V. VI. VII. VIII. IX. Introduction to the Paper Industry . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . The Papermaking Process . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Starch Consumption by the Paper Industry . . . . . . . . . . . . . . . . . . . . . . . . Starches for Use in Papermaking . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1. Current Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2. Recent Trends. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Application Requirements for Starch. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1. Viscosity Specifications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2. Charge Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3. Retrogradation Control . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4. Purity Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Dispersion of Starch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1. Delivery to the Paper Mill . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2. Suspension in Water. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3. Dispersion Under Atmospheric Pressure. . . . . . . . . . . . . . . . . . . . . . . . 4. Dispersion Under Elevated Pressure . . . . . . . . . . . . . . . . . . . . . . . . . . . 5. Chemical Conversion . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6. Enzymic Conversion . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Use of Starch in the Papermaking Furnish . . . . . . . . . . . . . . . . . . . . . . . . . 1. The Wet End of the Paper Machine . . . . . . . . . . . . . . . . . . . . . . . . . . . 2. Flocculation of Cellulose Fibers and Fines . . . . . . . . . . . . . . . . . . . . . . 3. Adsorption of Starch on Cellulose and Pigments . . . . . . . . . . . . . . . . . 4. Retention of Pigments and Cellulose Fines . . . . . . . . . . . . . . . . . . . . . . 5. Sheet Bonding by Starch. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6. Wet-end Sizing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7. Starch Selection for Wet-end Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Use of Starch for Surface Sizing of Paper. . . . . . . . . . . . . . . . . . . . . . . . . . 1. The Size Press in the Paper Machine . . . . . . . . . . . . . . . . . . . . . . . . . . . 2. The Water Box at the Calender. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3. Spray Application of Starch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4. Starch Selection for Surface Sizing . . . . . . . . . . . . . . . . . . . . . . . . . . . . Use of Starch as a Coating Binder . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1. The Coater in the Paper Machine . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2. Starch Selection for Paper Coating . . . . . . . . . . . . . . . . . . . . . . . . . . . . Starch: Chemistry and Technology, Third Edition ISBN: 978-0-12-746275-2 658 660 662 663 663 665 666 666 668 669 671 672 672 673 674 674 676 677 681 681 681 682 683 684 685 687 688 688 693 693 693 695 695 698 Copyright © 2009, Elsevier Inc. All rights reserved E-5 662 Starch in the Paper Industry pigments, binders and additives. Starch is a major coating binder. In one system roll coaters, a train of hydraulically loaded rolls, occasionally with a cell (gravure) pattern, are used. The coating color is metered by film splitting through a sequence of two or more nips prior to application to the paper. Current practice relies primarily on the use of various forms of blade coaters. The coating color is applied to paper by a pickup roll, an overflow device (SDTA) or a fountain (jet). A stiff (scraping) or a bent (gliding) blade is used to remove the excess. This process levels the coating on the sheet and generates a substrate for quality printing. In board coating, a pressurized air curtain (air knife) is often used to remove excess coating fluid from the sheet. In the newest technical development, a free-falling curtain of a coating is applied to the surface of paper or paperboard. Corrugating and laminating are subsequent converting processes for paper and board that require large quantities of starch. III. Starch Consumption by the Paper Industry Starch is an important component of many paper grades. Starch consumption by weight in papermaking and paper conversion processes ranks third after cellulose fiber and mineral pigments. Starch is used as a flocculant and retention aid, as a bonding agent, as a surface size, as a binder for coatings and as an adhesive in corrugated board, laminated grades and other products.14 Current consumption of industrial corn starch for paper and paperboard production in the US exceeds 2.5 billion pounds (1.1 million metric tons) of which 40% is chemically modified. Another 750 million pounds are used for corrugated and laminated paper products.15 Data for starch use are summarized in Table 18.2. The shipment reports of CRA, the Corn Refiners Association,15 are the main source for starch consumption data. Table 18.2 North American demand for starch in the manufacture of paper products Application Starch grade Actual use 1995a Projected for 2000a Wet endb Corn starch Potato starch Unmodified starch Oxidized starch Hydroxyethylated starch Cationic starch Unmodified starch Oxidized starch Hydroxyethylated starch Unmodified starch Modified starch 309 287 819 718 735 118 212 55 275 899 134 424 349 839 703 1034 137 203 81 340 Size pressc Coatingc Corrugating and laminatingd a Million pounds b Cationic, anionic or amphoteric c All corn starch d 1994 demand, members of CRA only Reprinted by permission of TAPPI E-6 666 Starch in the Paper Industry A previous trend in the paper industry of limiting starch purchases to unmodified grades and effecting modification on-site in the paper mill has changed. The variance in products thus obtained was frequently wider than in products supplied by the starch manufacturer. As a result, there is now more preference to utilize modified starches with specific application properties. Growth in paper recycling should lead to an increased use of starch as a coating binder in place of synthetic materials. New starch products might be derived from emulsion copolymerization with synthetic monomers and the replacement of all-synthetic polymers. Potential applications could be in flocculation, sizing, modified rheological characteristics, bonding to a wide range of substrates, film formation and in effluent treatment. A critical requirement will be the removal of hazardous residuals and Food and Drug Administration (FDA) approval for use in specific paper grades. Introduction of new starch products will require extensive technical services, especially for adaptation to closed paper machine wet-end systems, for use with deinked pulp and for the high shear conditions of high-speed paper coating.46 V. Application Requirements for Starch Dispersions of starch have found wide use in papermaking and paper conversion due to their unique properties, viz., low-cost renewable adhesive, controlled viscosity, specific rheological characteristics, water-holding properties, electrostatic charge, film formation and bonding after drying. Starches are chemically or physically modified to obtain specific properties of viscosity, charge, bonding to fibers and pigments, and bond strength. The viscosity of a dispersion of starch depends on concentration, chemical substitution on the starch molecule and molecular weight.47 Natural starch has a slight anionic electrostatic charge. The charge can be modified by chemical substitution that introduces anionic and/or cationic ionizing moieties and generates a specific charge or amphoteric property. Film-forming and bonding properties depend on molecular weight, the state of the starch dispersion and its water-holding properties. Improvements are obtained by chemical substitution. Modified starches, however, are only moderately different in their abilities to provide bonding strength and elongation. Humidity (moisture content) affects the strength and elongation of starch films and is often a dominating factor. Increasing humidity from 35% to 65% may decrease film strength by more than 40%.48 Various starch products are, therefore, distinguished more by rheological and charge characteristics than by bonding strength. Trade associations of the starch, paper, and agriculture industries have defined standard analytical methods for starch characterization. 1. Viscosity Specifications Starch is a natural product and as such is not uniform. Type, genetic variety and environmental factors of soil quality and weather during the growing season for the starch source may influence the rheological characteristics of the product. Additional E-7 Wheat Starch: Production, Properties, Modification and Uses 10 C.C. Maningat,1 P.A. Seib,2 S.D. Bassi,3 K.S. Woo4 and G.D. Lasater5 1 MGP Ingredients Inc., Atchison, Kansas, USA Department of Grain Science and Industry, Kansas State University, Manhattan, Kansas, USA 3 MGP Ingredients Inc., Atchison, Kansas, USA 4 MGP Ingredients Inc., Atchison, Kansas, USA 5 MGP Ingredients Inc., Atchison, Kansas, USA 2 I. Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . II. Production . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . III. Industrial Processes for Wheat Starch Production . . . . . . . . . . . . . . . . . . . 1. Conventional Processes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2. Hydrocyclone Process (Dough–Batter) . . . . . . . . . . . . . . . . . . . . . . . . . 3. High-pressure Disintegration Process . . . . . . . . . . . . . . . . . . . . . . . . . . IV. Properties of Wheat Starch and Wheat Starch Amylose and Amylopectin . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1. Large Versus Small Granules . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2. Fine Structures of Amylose and Amylopectin. . . . . . . . . . . . . . . . . . . . . 3. Partial Waxy and Waxy Wheat Starches . . . . . . . . . . . . . . . . . . . . . . . . 4. High-amylose Wheat Starch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5. A Unique Combination of Properties . . . . . . . . . . . . . . . . . . . . . . . . . . V. Modification of Wheat Starch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1. Crosslinking. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2. Substitution . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3. Dual Derivatization . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4. Bleaching, Oxidation and Acid-thinning . . . . . . . . . . . . . . . . . . . . . . . . VI. Uses of Unmodified and Modified Wheat Starches . . . . . . . . . . . . . . . . . . 1. Role in Baked Products . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2. Functionality in Noodles and Pasta. . . . . . . . . . . . . . . . . . . . . . . . . . . . 3. Other Food Uses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4. Industrial Uses. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . VII. References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Starch: Chemistry and Technology, Third Edition ISBN: 978-0-12-746275-2 442 442 444 446 448 450 451 452 457 465 470 471 475 475 478 479 480 481 481 485 488 489 491 Copyright © 2009, Elsevier Inc. All rights reserved E-8 VII. References 491 rigid as to resist writing pressure. Large granule wheat starch (sometimes called calibrated wheat starch) provided the best results for optimal functionality as a stilt material, because of the appropriate granule size and smooth surface. Thermoresistant large granule wheat starch (presumably highly crosslinked) is required for modern high-speed coaters running at 700–800 meters/minute and equipped with infrared driers to provide intensive drying due to a short residence time in the drying section of the coaters.204 One of the primary industrial applications of pregelatinized wheat starch is in the oil field services industry to thicken drilling fluids.4–6 In the building industry, both modified and unmodified wheat starches in pregelatinized form are used to texturize wall and ceiling coatings, as bonding agents in joint compounds for embedding joint tape, and in finishing gypsum panel joints, nail heads and metal corner beads.28 Starch in a glassy state was prepared from wheat starch by twin-screw extrusion for use as an environmentally-friendly abrasive grit to remove paints from aircraft surfaces.608–611 Another application of wheat starch is in cosmetics, where it was found to be non-toxic, non-irritating, non-sensitizing, and functional.612,613 Starch, in general, is useful in cosmetic powders because of its small size, enormous surface area, mobility, porosity, slip property and absorptive capacity.613 Wheat starch can enhance the softness and smoothness of face and body powders.614 Several modified wheat starches have been successfully formulated in creams, lotions, depilatories, hair relaxers, liquid make-up and cosmetic powders. Interest has been increasing in recent years to develop degradable plastics from starch, especially for disposable applications.615–621 The early efforts in the 1970s focused on using starch granules as fillers.589,593 More recent developments include thermoplastics that are intimate blends of starch molecules with hydrophilic vinyl polymers, such as poly(ethylene-co-acrylic acid) and poly(ethylene-co-vinyl alcohol), and with poly(ethylene glycol), polylactic acid and polycaprolactone622–628 (see Chapter 19). The function of the compatibilizer and the relationship of composition and morphology to mechanical properties of starch–polyolefin blends are the subject of several studies.629–633 Rigid and flexible foams, films and cushioning materials containing starch have also been developed.634–649 Native and modified wheat starches have been included in these investigations. VII. References 1. Olsen BT. In: Bushuk W, Rasper VF, eds. Wheat: Production, Properties and Quality. London, UK: Chapman and Hall; 1994 [Chapter 1]. 2. Olewnik MC. In: Chung OK, Lookhart GL, eds. Third International Wheat Quality Conference. Manhattan, KS: Grain Industry Alliance; 2005 [Session I]. 3. Radley JA. Starch and Its Derivatives. London, UK: Chapman and Hall; 1968. 4. Knight JW. Wheat Starch and Gluten. London, UK: Leonard Hill; 1965. 5. Knight JW. The Starch Industry. New York, NY: Pergamon Press; 1969. 6. Knight JW, Olson RM. In: Whistler RL, BeMiller JN, Paschall EF, eds. Starch: Chemistry and Technology. New York, NY: Academic Press; 1984 [Chapter 15]. E-9 Proceedings of The 9th Joint Conference on Chemistry ISBN 978-602-285-049-6 The Effects of Hydrolysis Temperature and Catalyst Concentration on Bio-ethanol Production from Banana Weevil Eni Budiyatia and Umar Bandia Abstract An energy need of petroleum fuels in various countries in the world in recent years has increased sharply. It doesn’t only happen in the developed countries but also in developing countries, including Indonesia. Scientists have a develop a renewable energy source to anticipate the crisis of petroleum fuels. Several types of renewable energy are biomass, geothermal, solar energy, water energy, wind energy, and ocean energy. Ethanol is a bio-fuel, and has good prospects as a substitute for liquid fuel and gasohol with renewable raw materials and environmentally friendly. Four steps are applied in this study. The first is preparation of tools and raw materials. All instruments were sterilized and banana weevil as raw material is cut and grind. The second is hydrolysis process, which HCl is used as catalyst. in the process,temperature are varied at 70 °C, 80 °C, 90 °C, and concentration of catalyst are 0.1; 0.2; and 0.3 N. The third step is fermentation, which is conducted at the ambient temperature (27 °C) and anaerobic conditions. The last is distillation process. The results show the greater hydrolysis temperature, the concentration and the yield of produced bio-ethanol greater. The hydrolysis process of HCl 0.3 N at 90 °C, resulted in the greatest level of bio-ethanol, which is 61.20%. This research should be developed, especially for the purification process on order to obtain higher ethanol concentration. aChemical Engineering Program – Muhammadiyah University of Surakarta, A. Yani Street Tromol Pos I Pabelan Kartasura Surakarta–Indonesia Corresponding author e-mail address: [email protected] and [email protected] Introduction Banana (Musa paradisiacal) Ethanol is a bio-fuel, has good prospects as a substitute for liquid fuel and gasohol with renewable raw materials, environmentally friendly and very beneficial economically for rural communities, especially farmers. According to the Energy Minister's decision No. 32 of 2008 "bio-ethanol (E100) is product of ethanol produced from biological raw materials and other processed biomass in biotechnology and shall meet the quality standard (specification) in accordance with the provisions of the legislation to be used as alternative fuel ". Banana (Musa paradisiacal) is an herbaceous fruit plants originating from areas in Southeast Asia. These plants then spread to Africa (Madagascar), South and Central America. Bananas in West Java called “cau”, in Central Java and East Java called “gedang”. Banana plants can be easily found almost in every place. Banana production centre in West Java is Cianjur, Sukabumi and the area around Cirebon. Bananas are generally able to grow in the lowlands to the mountains with an altitude of 2000 m. Bananas can grow on wet tropical climate, humid and hot with optimum rainfall is 1520-3800 mm/year with 2 months to dry (Rismunandar, 1990). This study used banana weevil as raw material for bioethanol production because the banana weevil has a composition of 76% starch, 20% water, and the rest is protein and vitamins (Yuanita et al., 2008). The benefit to the community is this process can reduce banana plant waste, especially banana weevil. Besides that, it can be used to raise the added value of banana weevil into valuable chemical. Industry of ethanol in Indonesia can use banana weevil as an alternative to the manufacture bio-ethanol, as a reference and development potential hump banana biomass as a feedstock for bio-ethanol production. Banana weevil can be used to be taken the starch, this starch resembling sago starch flour and tapioca flour. The potential content of banana weevil starch can be used as an alternative fuel that is, bio-ethanol. Starchy materials are used as raw material for bio-ethanol suggested that high levels of starch, has a high yield potential, flexible in farming and harvesting (Prihandana, 2007 and Aswandi et al., 2012). Green Chemistry Section 2: Physical Chemistry, Eni Budiyati, et al. P a g e | 161 This Proceedings©Chemistry Department, FSM, Diponegoro University 2015 E-10 Proceedings of The 9th Joint Conference on Chemistry ISBN 978-602-285-049-6 Table 1. Chemical content of 100 gram banana weevil No Component Wet Dry 1 Starch (gram) 96 76 2 Calories (cal) 43 425 3 Protein (gram) 0.6 3.4 4 Carbohydrates (gram) 11.6 66.2 5 Ca (mg) 15 60 6 P (mg) 60 150 7 Fe (mg) 1 2 8 Vitamin C (mg) 12 4 Bio-ethanol Bio-ethanol production is determined by: 1) number of raw material, 2) the amount of sugar that ready to be fermented, and 3) efficiency of fermentation process to convert sugar into alcohol (Smith, et al., 2006). Bioethanol is ethanol (ethyl alcohol) produced using natural raw materials and biological processes. Ethanol is used as a vehicle fuel has a chemical structure that is identical to that found in ethanol liquor. Ethanol used for fuel called by Fuel Grade Ethanol (FGE) with a purity level of 99.5%. Ethanol is an organic compound composed of carbon, hydrogen and oxygen. So it can be viewed as derivatives of hydrocarbon compounds having a hydroxyl group with the formula C2H5OH (Hendroko, 2008). b. : 46.07 g/mol : No Colour : Liquid : 78.4 °C : -112 °C : 0.7893 : Infinity :Infinity 2007) (Perry, Chemical properties of ethanol Burning ethanol produces carbon dioxide and water: C2H5OH (g) + 3 O2 (g) → 2 CO2 (g) + 3 H2O (l) Ethanol can be used as an automotive fuel is varied, from blend to pure bio-ethanol. Bio-ethanol is often referred to by the notation "Ex", where x is the percentage of ethanol content in the fuel. Some examples of the use of the notation "Ex" are: 1. 2. 3. E100, 100% bio-ethanol or without a mixture E85, a blend of 85% bio-ethanol and 15% petrol E5, a mixture of 5% bio-ethanol and 95% petrol 162|P a g e Hydrolysis is a process of the reactants with water to break compound. in the hydrolysis of starch with water, the water will attack the starch on α 1-4 glucosidal bond form dextrin, syrup or glucose depending on the degree of starch breakdown in the polysaccharide chain. Reaction is first order reaction if excess water is used, so that changes of reactants can be ignored. The reaction between water and starch goes so slowly, so it needs a catalyst to increase the reactivity of water. This catalyst can be acidic, alkaline or enzyme (Coney, 1979 in Retno 2009). Hydrolysis process of starch into sugars is required following reaction: (C6H10O5) n + nH2O Polysaccharides Water a. Physical properties of ethanol Molecular weight Colour Phase The normal boiling point Freezing Point Specific Gravity Solubility in 100 parts Water Other reagents Hydrolysis → n(C6H1206) Glucose The Influence variables of the hydrolysis reaction: Characteristics of ethanol: a. PERTAMINA has sold bio-premium (E5) containing 5% bio-ethanol and 95% premium. E5 fuel can be used on vehicles that use petrol (gasoline) standard, without any modification. However, E15 fuel up or a percentage of more than 15% ethanol must utilize the vehicle with the type of Flexible-Fuel Vehicle. Brazil as one of the countries that use the world's largest bioethanol, has adopted the E100 fuel, which contains 100% bio-ethanol (Atmojo, 2010). Catalyst Almost all of the hydrolysis reaction requires a catalyst to accelerate the reaction. The catalyst used can be either enzymes or acid. The acids usually used are hydrochloric acid (Agra et al., 1973; Stout & Rydberg Jr., 1939 in Prasetyo, 2011), sulphuric acid, and nitric acid. The H ion concentration give bigger affect to reaction rate than the type of acid. Nevertheless, generally the industry use hydrochloric acid. This selection was based on the salt formed in neutralization reaction. Sodium chloride is safe and there is dangerous when the concentration is too high (just give salty taste). So, the acid concentration in water is controlled. Commonly acid solution concentration that used has a higher than concentration of acid in the manufacture of syrup. Hydrolysis at a pressure of 1 atm requires a much more concentrated acid. The rate of hydrolysis process will increase by a high concentration of acid. in addition to adding the rate of hydrolysis process, a high concentration of acid will also result in binding of ions such as SiO2 controller, phosphate, and salts such as Ca, Mg, and Na in starch. Therefore, the appropriate comparison is required between the starch will be hydrolysed to the acid concentration (Sun and Cheng, 2005). Green Chemistry Section 2: Physical Chemistry, Eni Budiyati, et al. This Proceedings©Chemistry Department, FSM, Diponegoro University 2015 E-11 Proceedings of The 9th Joint Conference on Chemistry b. Temperature and pressure The influence of temperature on the reaction rate follows Arrhenius equations. The higher temperature will increase the reaction rate. for example, to achieve a certain conversion takes about 3 hours to hydrolyse starch sweet potatoes at 100 °C. But if the temperature is raised to a temperature of 135 °C, the reach the same conversion can be reached in 40 minutes (Agra et al., 1973 in Prasetyo, 2011). Wheat and corn starch hydrolysis with sulphuric acid catalyst requires a temperature of 160 °C. Since the heat of reaction is almost close to zero and the reaction in the liquid phase, the temperature and pressure are not much affect the balance. c. Mixing (stirring) Reaction rate will be faster if reactants can collide with each other as well as possible, so mixing is needed. for a batch process, this can be achieved by use stirrer or shaker (Agra et al., 1973 in Prasetyo, 2011). If the process is a process flow (continuous), then the mixing is done by regulating the flow in the reactor in order to increase turbulence. d. Comparison of reagents If one of the reactants is excessive amount then the balance may shift to the right as well. Therefore, low levels of starch suspension may give better results than high starch levels. If levels of suspense lowered from 40% to 20% or 1%, then the conversion will increase from 80% to 87 or 99% (Groggins, 1958). At the surface level, high starch suspense molecules will be difficult to move. The five types of hydrolysis, namely: a. Hydrolysis in Acid Solution Dilute or concentrated acid such as HCl, H2SO4 (other expensive acid) are usually serves as a catalyst. in dilute acid, commonly, the reaction rate is proportional to the concentration of H or [H+]. These properties do not apply to concentrated acid. High sugar efficiency recovery as well as the potential for cost reduction, is most significantly the advantage of the acid hydrolysis process (Matz, 1970 in Retno, 2009). The weakness of the acid hydrolysis is degradation of sugars results in the hydrolysis reaction and the formation of undesired products. Degradation of sugar and side product will not only reduce the sugar harvest, but side product also can inhibit the formation of ethanol in the next fermentation stage. b. Bases in the hydrolysis solution Dilute or concentrated bases used in the hydrolysis reaction are NaOH and KOH. Result of this process is not acid but salt. Two main advantages of this method ISBN 978-602-285-049-6 are the reaction is occur irreversible and its products more easily to be separated. However, a potential problem regarding the disposal of waste is the disadvantage of this process. c. Hydrolysis with enzyme as catalyst (Retno et al, 2011) An α-amylase is one of enzymes produced by microbes. The advantage of enzymatic hydrolysis is able to degrade complex carbohydrates into simple sugars with more results. However, enzymatic hydrolysis also has some weaknesses such as low hydrolysis rate and expensive cost. d. Pure hydrolysis Reacted with H2O without catalyst, the reaction is slow so rarely used in the industry. This process is suitable for reactive compounds. The reaction can be accelerated by using H2Ovapour. e. Alkali Fusion Either with or without H2O at high temperatures, e.g. in solid NaOH (H2O<<). Usage in the industry for a specific purpose, such as smelting cellulosic materials such as corn cobs, “grajen” wood performed at high temperature (± 240 °C) with solid NaOH produces oxalic acid and acetic acid. Fermentation Ethanol fermentation, referred to as alcoholic fermentation, is a biological process in which sugars such as glucose, fructose, and sucrose are converted into cellular energy and also produce ethanol and carbon dioxide as by-products. This process does not require oxygen, then the ethanol fermentation is classified as anaerobic respiration. Fermentation ethanol is used in the manufacture of alcoholic beverages, ethanol fuel, and added agent in bread cooking. The types of fermentation are: 1. Alcoholic fermentation Alcoholic fermentation is a conversion reaction of glucose to ethanol (ethyl alcohol) and carbon dioxide. 2. Lactic acid fermentation Lactic acid fermentation is that respiration occurs in animals or human cells, when the oxygen requirement is not fulfilled due to overwork. in the muscle cells, lactic acid can cause symptoms of cramps and fatigue. Lactate accumulated as waste products can cause Green Chemistry Section 2: Physical Chemistry, Eni Budiyati, et al. P a g e | 163 This Proceedings©Chemistry Department, FSM, Diponegoro University 2015 E-12 Jurnal Penelitian Pertanian Terapan Vol. 18 (1): 52-58 http://www.jurnal.polinela.ac.id/JPPT DOI: http://dx.doi.org/10.25181/JPPT.V18I1.1043 pISSN 1410-5020 eISSN 2047-1781 Analisis Karakteristik Kimia Tepung Kasava dari Ubikayu Varietas Klenteng dan Casessart (UJ5) Analysis of Chemical Characteristic of Casava Flour from Klenteng and Casessart (UJ5) Varieties Erliana Novitasari* dan Ratna Wylis Arief Balai Pengkajian Teknologi Pertanian (BPTP) Lampung * Email : [email protected]; [email protected] ABSTRACT The technology of cassava flour modification has been researched and developed. Biological change by using BIMO-CF containing lactic acid bacteria is a practical technology that is easy to apply in the production of cassava flour. This research was conducted from May until August 2017 at Agrosains Park Natar with the aim to know the chemical characteristics of cassava flour from Klenteng and Casessart varieties. Observation parameters included analysis of moisture content, ash content, fat content, protein content, fiber content, total carbohydrate content, starch content, HCN content, and white degree at THP Polytechnic State Laboratory of Lampung. The results showed that the highest yield was produced by cassava flour from casessart variety with the addition of BIMO-CF were 23.11%. The water content of cassava flour produced ranged between 8.02-9.19%, by the quality requirements of SNI. The lowest ash content was cassava flour from casessart variety (1.19%) without the addition of starter. The addition of starter increased the protein content of cassava flour both of Klenteng variety (0.47%) and Casessart variety (1.11%), decreasing the fiber content for Klenteng variety (0.67%) and Casessart variety (0.90%). The amount of fat contained in cassava flour produced ranged from 0.69 to 0.87%. Carbohydrate content (Klenteng variety was 88.49%, and Casessart variety was 87,69%) and starch content (Klenteng variety was 85,98%, and Casessart variety was 84,83%), cassava flour with the addition of starter higher than cassava flour without the addition of starter. All of the cassava flour produced has HCN levels below the maximum limit (0.0216-0.0293%), while the degree of white (> 80%) has not met the quality requirements of SNI. Keywords: chemical characteristics, cassava flour, varieties Disubmit: 24 Desember 2017, Diterima: 20 Januari 2018, Disetujui: 31 Januari 2018 PENDAHULUAN Kasava atau biasa disebut ubi kayu atau singkong merupakan salah satu komoditas tanaman pangan non beras unggulan di Provinsi Lampung. Pada tahun 2017 tercatat produksi ubi kayu di Provinsi Lampung sebesar 7.387.084 ton dari lahan tanam seluas 279.337 hektar dengan produktivitas 26,44 ton/ hektar (Badan Pusat Statistik, 2018). Ubi kayu mempunyai potensi untuk dimanfaatkan sebagai alternatif sumber pangan pokok untuk mendukung program ketahanan pangan. Tahun 2016 Indonesia tercatat sebagai rangking empat negara penghasil ubi kayu di dunia setelah Nigeria, Thailand dan Brazil (FAO, 2017). Tetapi tidak dapat dipungkiri bahwa Indonesia masih menghadapi masalah besar yaitu ketergantungan terhadap sebagian bahan pangan impor yaitu gandum. E-13 Jurnal Penelitian Pertanian Terapan lain proses fotosintesis pada tanaman tersebut. Kandungan terbesar dalam ubikayu adalah air dan karbohidrat yang merupakan sumber utama energi (Salvador, Steenkamp, & Mccrindle, 2014). Tabel 3. Kandungan karbohidrat dan pati Perlakuan Karbohidrat (%) Kadar pati (%) Klenteng 85,73 84,53 Klenteng + starter 88,49 85,98 Casessart 87,49 74,52 Casessart + starter 87,69 84,83 * Sumber: BSN (1996) dalam Yulifianti et al., (2012) Pati merupakan salah satu bentuk dari karbohidrat jenis polisakarida. Alam menyediakan polisakarida yang banyak ditemukan pada tanaman. Proses fotosintesis menghasilkan karbohidrat yang tersimpan dalm bentuk pati. Pati atau amilum mempunyai sifat tidak larut dalam air pada suhu kamar dan tidak berasa maupun berbau. Pati tersusun atas dua macam polimer polisakarida, yaitu amilopektin dan amilosa dalam perbandingan yang bermacam-macam (Ariani et al., 2017). Pati dengan kandungan amilosa tinggi lebih mudah larut dalam air karena memiliki banyak gugus hidroksil sehingga sulit membentuk gel dan sulit mengental. Sedangkan pati dengan kandungan amilopektin tinggi memiliki sifat mengembang lebih baik dibandingkan amilosa (Kusnandar, 2010 dalam Ariani et al., 2017). Selain itu, pati dengan kandungan amilosa tinggi bersifat kurang rekat dan kering dibandingkan pati yang memiliki kandungan amilopektin tinggi yang bersifat rekat dan basah (Hidayat, Ahza, & Sugiyono, 2007). Hasil analisa kadar pati tepung ubi kayu varietas Klenteng dan Casessart menunjukkan kadar sebesar 74,52-85,98%. Kadar HCN dan Derajat Putih Hasil pengujian terhadap kandungan HCN atau asam sianida dan derajat putih tepung kasava disajikan pada Tabel 4. Analisis HCN dilakukan untuk mengetahui kadar asam sianida pada tepung ubikayu setelah direndam selama 24 jam. Hasil menunjukkan bahwa tepung ubikayu yang telah melewati tahap perendaman selama 24 jam hanya mengandung HCN 21,6-29,3 mg/kg. Dalam bentuk umbi segar, kandungan HCN singkong makan/ tidak pahit seperti Klenteng, Adira 1 dan Malang 1 mempunyai kadar HCN maksimal 40 mg/kg, sedangkan untuk ubikayu pahit seperti Casessart atau UJ 5, UJ 3 (Thailand), Adira 4 mempunya kadar HCN lebih dari 100 mg/kg (Balitkabi, 2011). Kadar HCN dalam tepung kasava berkurang karena telah melalui proses perendaman. Hal ini sesuai dengan hasil terdahulu yang menyatakan bahwa turunnya kadar HCN akibat hidrolisis dinding sel mikroba selama proses fermentasi. Penambahan starter mempercepat menurunnya tingkat kekerasan umbi dan meningkatkan nilai keasaman media menginaktivasi enzim linamarase sehingga tidak dapat membentuk HCN (Nkoudou & Essia, 2017). Derajat putih tepung merupakan parameter yang penting karena mempengaruhi performansi hasil akhir produk tepung tersebut. Hasil pengujian derajat putih menunjukkan bahwa tepung berkisar antara 74,55-79,75% seperti tersaji pada Tabel 4. Tepung ubikayu varietas Klenteng tanpa penambahan starter menunjukkan level derajat putih yang tertinggi dan varietas Casessart dengan penambahan starter menunjukkan tingkat derajat putih paling rendah. Menurut Yulifianti et al. (2012) perendaman dan pencucian selain bertujuan untuk melunakkan tekstur ubi kayu, juga dapat membersihkan kontaminan yang menyebabkan warna selain putih. Fermentasi dapat menghilangkan komponen penimbul warna, seperti pigmen pada umbi yang berwarna kuning dan protein yang mengakibatkan warna kecoklatan. Hal 56 Volume 18, Nomor 1, 2018 E-14 Scientia Agroalimentaria ISSN: 2339-4684 Vol. 1 (2013) 19-25 PHYSICOCHEMICAL CHARACTERIZATION OF TWO CASSAVA (Manihot esculenta Crantz) STARCHES AND FLOURS CARACTERIZACIÓN FISICOQUIMICA DE DOS ALMIDONES Y HARINAS DE YUCA (Manihot esculenta Crantz) Sandoval Aldana, A.1; Fernández Quintero, A.2 Resumen El almidón y la harina de yuca fueron obtenidos de raíces cultivadas en Colombia en dos condiciones ambientales específicas. Se evaluaron propiedades fisicoquímicas como tamaño y morfología del grano, contenido de amilosa, cristalinidad, propiedades térmicas y comportamiento al empastamiento. Las propiedades del almidón de yuca fueron altamente influenciadas por las condiciones ambientales durante el periodo de crecimiento de las raíces de yuca. El almidón extraído de las raíces de yuca cultivadas en una zona con temperatura promedio más alta presentó un tamaño de granulo más pequeño, mayor contenido de amilosa y mayor temperatura y entalpia de gelatinización, lo que está relacionado con una mayor temperatura de empastamiento y menor viscosidad. Las harinas de yuca presentaron diferencias con los almidones estudiados como una menor entalpia de gelatinización medida por calorimetría diferencial de barrido (DSC), mayor temperatura de empastamiento y menor desarrollo de viscosidad máxima. Este comportamiento posiblemente esta influenciado por la presencia de otros componentes diferentes al almidón en la raíz de yuca fresca. Palabras clave: almidón, harina, yuca, DSC, rayos X, empastamiento Abstract Starch and flour were produced from cassava roots grown in two specific environmental conditions in Colombia. The physicochemical properties evaluated were granule size and morphology, amylose content, crystal form, thermal properties and pasting behavior. The properties of cassava starch were highly influenced by the environmental conditions during the growth of the roots. Starch extracted from roots cultivated in a warmer zone showed smaller granule size, higher amylose content and higher temperature and enthalpy of gelatinization. This starch also showed higher pasting temperature and lower peak viscosity. Cassava flours presented differences with their corresponding starch such as lower enthalpy of gelatinization measured by DSC, higher pasting temperature and lower peak viscosity on pasting. It is possible that this behavior is influenced by the presence of non-starch components from the fresh root. Keywords: starch, flour, cassava, DSC, X-ray, pasting behavior. 1 2 Profesor, Facultad de Ingeniería Agronómica, Universidad del Tolima; Barrio Santa Helena, A.A. 546, correo: [email protected] Profesor Titular, Departamento de Ingeniería de Alimentos, Facultad de Ingeniería, Universidad del Valle. Fecha de recepción: 17-12-2012 Fecha de aprobación: 04-02-2013 E-15 Sandoval-Aldana, A., et al. Scientia Agroalimentaria. Vol. 1 (2013)19-25 which the starch granules are synthesized [21, 7]. Besides, Noda et al. [23] have reported, in studies on sweet potato and wheat starches, that low values of gelatinization onset, peak and conclusion temperature measured by DSC, reflected the presence of abundant short amylopectin chains. The values of temperature and enthalpy of gelatinization determined in this study were higher 23 than the values reported by Asaoka et al. [4] for Colombian varieties, temperature of gelatinization 50.7-57 oC and enthalpy 7-9 J g-1. The enthalpy of gelatinization for starch A was in agreement with the value reported by Charles et al. [1] for Thai cassava starch and for Indian starch [12]. Higher value of enthalpy as starch B has been reported by Abera and Rakshit [24]. Table 2. DSC thermal properties of cassava starches and flours.1 Material DSC onset (°C) DSC peak (°C) 60.69 ± 0.53 65.44 ± 0.51 Starch A 66.51 ± 0.24 72.00 ± 0.44 Starch B 60.99 ± 0.71 66.14 ± 0.33 Flour A 66.01 ± 0.41 71.00 ± 0.22 Flour B 1 The results are means of three experiments and standard deviation. The results of gelatinization temperature for the flours were closer to the values determined for their corresponding starch, this contrasts with the results of Moorthy et al. [12] and Defloor et al. [25]. They postulated that the presence of non-starch components, which competed for the available water, delayed the gelatinization. Despite the high crude fiber content for flour B, the temperature of gelatinization was the same as in the starch. These differences could be related to crude protein content as well as environmental conditions. Pereira and Beleia [10] reported that cassava flours from peeled roots presented higher protein content which decreased with the age of the roots. Besides, Defloor et al. [23] stated that gelatinization temperature of cassava flour increased as roots were grown in dry conditions. Root age at harvest, protein content and moisture stress were not reported by Moorthy et al. [12]. The enthalpy of gelatinization for flour samples presented significant differences (P < 0.001) with the enthalpy of the starch. This behavior was reported before by Moorthy et al. [12]. Cassava flour gelatinization enthalpy was in the range of 7-8 J g-1, these values were lower than those reported for Indian varieties (9-10 J g-1). The RVA results are presented in Table 3. Peak viscosity and final viscosity values of cassava starches showed significant differences (P<0.001). Starch A showed a lower pasting temperature and developed higher viscosity. Differences in pasting behavior between starches were observed before by FernándezQuintero [5], who stated that starches from plants of zone B presented higher initial pasting temperature and exhibited lower viscosities on pasting. DSC end (°C) 71.36 ± 0.98 79.26 ± 0.33 69.91 ± 0.14 77.22 ± 0.59 Enthalpy ∆ H (J g-1) 13.96 ± 0.06 15.50 ± 0.74 7.75 ± 0.07 8.11 ± 0.64 Table 3. RVA pasting results for cassava starches and flours. Material Peak viscosity (m∙Pa∙s) Trough viscosity (m∙Pa∙s) Final viscosity (m∙Pa∙s) Onset temperature (°C) Starch A 5716 2367 2697 67.85 Starch B 4862 2670 3085 74.35 Flour A 4325 2640 3080 71.25 Flour B 3100 2580 3210 75.95 The size and size distribution of starch granules might contribute in the pasting behavior and the rheological response of starches and swelling of granules [26, 27]. Starch from Zone B presented a higher proportion of small granules than starch from zone A. The differences in the granule size between the samples could partially explain their different behavior during pasting. Starch B also presented a higher value of final viscosity. Charles et al. [1] and Sriroth et al. [6] reported that starch with high amylose content developed high final viscosity and setback on pasting. Pasting profiles for cassava starches and flours are plotted in Figure 3 and Figure 4. There were significant differences (P < 0.001) in the pasting profiles between starches and their corresponding flours. Lower peak viscosity values and higher pasting temperatures were obtained for both flours. This lower viscosity values could be partly attributed to lower starch content. It is also possible that the minor components (protein and fiber) influenced the values of viscosity on pasting [11, 12]. E-16 24 Sandoval-Aldana, A., et al. Scientia Agroalimentaria. Vol. 1 (2013) 19-25 6000 100 90 80 4500 70 3750 60 3000 50 2250 40 Temperature (ºC) Viscosity (mPas) 5250 30 1500 Starch Flour 750 20 10 0 0 0 200 400 600 800 Time (s) Figure 3. Pasting behavior for cassava starch and flour A. Pasting profiles for cassava starches and flours are plotted in Figure 3 and Figure 4. There were significant differences (P < 0.001) in the pasting profiles between starches and their corresponding flours. Lower peak viscosity values and higher pasting temperatures were obtained for both flours. This lower viscosity values could be partly attributed to lower starch content. It is also possible that the minor components (protein and fiber) influenced the values of viscosity on pasting [11, 12]. 6000 100 90 80 4500 70 3750 60 3000 50 40 2250 30 1500 750 Starch B 20 Flour B 10 0 Temperature(ºC) Viscosity (mPas) 5250 0 0 200 400 600 Physicochemical properties of flour were influenced by chemical composition, which was a consequence of the procedure for obtaining the flours. The presence of non-starch components in the flours decreased the values of enthalpy of gelatinization and increased pasting temperature but decreased peak viscosity. 800 Time Figure 4. Pasting behavior for cassava starch and flour B. Conclusions The physicochemical properties of starches were highly influenced by the environmental conditions during the growing period of the plants. Small granule size, high amylose content, high temperature and enthalpy of gelatinization were characteristics of starch extracted from roots cultivated at high temperatures. These physicochemical properties are also related to functional properties as high pasting temperature and low peak viscosity. Therefore, it was confirmed that there are trends in the behavior of cassava starch from roots grown in a specific environmental condition. Acknowledgements A. Sandoval-Aldana was funded by COLCIENCIAS. The authors would like to thank Clayuca – CIAT and Industrias del Maíz S.A. for supplying the raw materials. References [1] Charles, A.L., Y.-H. Chang, W.-C.Ko, K. Sriroth, and T.-C. Huang.(2004). Some physical and chemical properties of starch isolates of cassava genotypes. Starch-Stärke, 56, 413418. [2] Glicksman, M. (1969). Staches., In M. Glicksman, ed. Gum technology in the food industry (Pp. 278). New York: Academic Press. [3] Asaoka, M., J.M.V. Blanshard, and J.E. Rickard. (1991). Seasonal effects on the physico-chemical properties of starch from four cultivars of cassava.Starch-Stärke 43, 455-459. [4] Asaoka, M., J.M.V. Blanshard, and J.E. Rickard. (1992). Effect of cultivar and growth season on the gelatinisation properties of cassava (Manihot esculenta) starch.Journal of the Science of Food and Agriculture,59, 53-58. [5] Fernández-Quintero, A. (1996). Effect of processing procedures and cultivar on the properties of cassava flour and starch.Ph. D. Thesis, University of Nottingham, Loughborough. [6] Sriroth, K., V. Santisopasri, C. Petchalanuwat, K. Kiurotjanwong, K. Piyachomkwan, and C.G. Oates. (1999). Cassava starch granule structure-function properties: influence of time and conditions at harvest on four cultivar of cassava starch. Carbohydrate Polymers,38, 161-170. [7] Tester, R.F. (1997). Starch: the polysaccharide fractions, In P. J. Frazier, P. Richmond, & A. M. Donald, (Eds.), Starch: structure and functionality (p. 163-171). Royal Society of Chemistry, Cambridge. [8] Badrie, N., and W.A. Mellowes. (1991). Effect of extrusion variables on cassava extrudates.Journal of Food Science, 56, 1334-1337. [9] Aryee, F.N.A., I. Oduro, W.O. Ellis, and J.J. Afuakwa. (2005). The physicochemical properties of flour samples from the roots of 31 varieties of cassava. Food Control.In press. [10] Pereira, L.T.P., and A.d.P. Beleia. (2004). Isolamento, fracionamento e caracterizacao de parees celulares de raizes de mandioca (Manihot esculenta Crantz). Ciencia y Tecnología Alimentaría, 24, 59-63. [11] Niba, L.L., M.M. Bokanga, E.L. Jackson, D.S. Schlimme, and B.W. Li. (2001). Physicochemical properties and starch granular characteristics of flour from various Manihot E-17 ISSN 0101-2061 Original Ciência e Tecnologia de Alimentos The effect of acid hydrolysis on the technological functional properties of pinhão (Araucaria brasiliensis) starch Efeito da hidrólise ácida nas propriedades funcionais tecnológicas do amido de pinhão (Araucaria brasiliensis) Roberta Cruz Silveira THYS1*, Andréia Gomes AIRES1, Ligia Damasceno Ferreira MARCZAK2, Caciano Pelayo Zapata NOREÑA1 Abstract Technological functional properties of native and acid-thinned pinhão (seeds of Araucária angustifolia, Brazilian pine) starches were evaluated and compared to those of native and acid-thinned corn starches. The starches were hydrolyzed (3.2 mol.L–1 HCl, 44 °C, 6 hours) and evaluated before and after the hydrolysis reaction in terms of formation, melting point and thermo-reversibility of gel starches, retrogradation (in a 30-day period and measurements every three days), paste freezing and thawing stability (after six freezing and thawing cycles), swelling power, and solubility. The results of light transmittance (%) of pastes of native and acid-thinned pinhão starches was higher (lower tendency to retrogradation) than that obtained for corn starches after similar storage period. Native pinhão starch (NPS) presented lower syneresis than native corn starch (NCS) when submitted to freeze-thaw cycles. The acid hydrolysis increased the syneresis of the two native varieties under storage at 5 °C and after freezing and thawing cycles. The solubility of NPS was lower than that of native corn starch at 25, 50, and 70 °C. However, for the acid-thinned pinhão starch (APS), this property was significantly higher (p < 0.05) when compared to that of acid-thinned corn starch (ACS). From the results obtained, it can be said that the acid treatment was efficient in producing a potential fat substitute from pinhão starch variety, but this ability must be further investigated. Keywords: unconventional starch source; modified starch; fat substitutes. Resumo As propriedades funcionais tecnológicas do amido nativo e modificado (hidrólise ácida) de pinhão (Araucaria angustifólia) foram comparadas às propriedades do amido nativo e ácido hidrolisado de milho. As espécies de amido foram hidrolisadas (3.2 mol.L–1 HCl, 44 °C, 6 horas) e avaliadas, antes e após a reação de hidrólise, de acordo com as análises de formação, fusão e termorreversão do gel, retrogradação (em um período de 30 dias, com medidas a cada 3 dias), estabilidade ao congelamento e descongelamento (após 6 ciclos de congelamento e descongelamento), poder de inchamento e índice de solubilidade. Os resultados obtidos demonstraram que o amido de pinhão apresenta menor tendência à retrogradação quando comparado ao amido de milho, tanto para a forma nativa quanto na modificada, após períodos similares de armazenamento. O amido nativo de pinhão (APN), quando submetido a sucessivos ciclos de congelamento e descongelamento, apresentou menor sinérese do que o amido de milho nativo (AMN). Nas temperaturas de 25, 50 e 70 °C, a solubilidade do APN foi menor do que a obtida pelo AMN. Entretanto, para a forma modificada, o amido de pinhão apresentou maior solubilidade (p < 0,05) do que o amido de milho. Através dos resultados, pode-se afirmar que o tratamento ácido modificado realizado no amido de pinhão foi efetivo para a produção de um potencial substituto de gordura, propriedade que deve ser testada e analisada em estudos futuros. Palavras-chave: fonte de amido não convencional; amido modificado; substituto de gordura. 1 Introduction Brazilian Pine (Araucaria brasiliensis syn. A. angustifolia) belongs to the Araucariaceae family and is the most economically important native conifer species in Brazil (ZANDAVALLI; DILLENBURG; DE SOUZA, 2004). The seed of this tree, harvested from April to August, is known as pinhão, and it is most commonly eaten after being cooked and peeled. Pinhão is also used as raw flour as an ingredient for several dishes, and is considered a source of starch (~36%), dietary fiber, magnesium and copper, besides producing a low glycemic index after its consume (CORDENUNSI et al., 2004). Although nutritional and technological aspects of pinhão are scarce in the scientific literature, recent studies suggest that the Araucaria seed is a potential alternative source of starch extraction for industrial purposes (CORDENUNSI et al., 2004; BELLO-PEREZ et al., 2006; STAHL et al., 2007). Starch is the most commonly thickening and gelling agent used by the food industry in the development of a large number of products such as soups, flans, sauces, and readyto-eat food among others. In recent years, there has been an effort of researchers to find new sources of unconventional native starch with the necessary properties for the food industry, such as absence of syneresis, transparency, stability, Received 22/6/2012 Accepted 16/10/2012 (00T5756) 1 Institute of Food Science and Technology – ICTA, Federal University of Rio Grande do Sul – UFRGS, Av. Bento Gonçalves, 9500, Campus do Vale, CEP 91501-970, Porto Alegre, RS, Brazil, e-mail: [email protected] 2 Department of Chemical Engineering, Federal University of Rio Grande do Sul – UFRGS, CEP 91501-970, Porto Alegre, RS, Brazil *Corresponding author Ciênc. Tecnol. Aliment., Campinas, 33(Supl. 1): 89-94, fev. 2013 89 E-18 Thys et al. Table 3. Solubility of native and acid-thinned starches from pinhão and corn. Temperature 25 °C 50 °C 60 °C 70 °C NPS 0.31aA ± 0.04 0.12aB ± 0.01 4.02aC ± 0.20 1.45aD ± 0.09 Variety NCS APS 0.71bA ± 0.01 7.52cA ± 0.12 1.48bB ± 0.04 7.47cA ± 0.12 2.98bC ± 0.11 8.65cB ± 0.02 2.85bC ± 0.06 9.02cC ± 0.05 ACS 6.87dA ± 0.07 6.93dA ± 0.14 8.11dB ± 0.04 8.19dB ± 0.02 The results are expressed as mean ± standard deviation (n = 3). Means followed by different lowercase letters in the same row indicate significant differences by Tukey test (p < 0.05). Means followed by different capital letters in the same column indicate significant differences by Tukey test (p < 0.05). harvest time also affect the swelling power (FRANCO et al., 2002), which could explain the first situation mentioned above. Man et al. (2012) reported that no significant difference between native and acid-thinned starch was found under 65 °C, but, at temperatures higher than 80 °C, the SP gradually decreased. The solubility (Table 3) of NPS was lower than that of NCS at 25, 50, and 70 °C, which can be explained by the lower amylose content of pinhão starch compared to that of corn starch. Amylose dissociates from the granule, which contributes to solubility increase (MARCON et al., 2007). Wosiacki and Cereda (1989) reported a similar pattern for pinhão starch at temperatures higher than 85 °C. Bello-Pérez et al. (2006) reported an inverse behavior, with higher solubility values for pinhão starch, which according to these authors is in agreement with the lower temperature and enthalpy of gelatinization of pinhão starch assessed by DSC in their study. APS and ACS showed higher values of solubility than the native starches. This occurs because the acid hydrolizes preferentially the amorphous region of the starch molecule , where amylose is normally found (ATICHOKUDOMCHAI et al., 2000), generating a significant reduction in the amylose chain length in the granule content, and its consequent dissolution resulting in solubility increase; fact that was evidenced with increases in temperature in the corn and pinhão species. In addition, APS had significantly higher (p < 0.05) solubility than ACS showing a higher susceptibility of pinhão starch to acid hydrolysis, when compared to that of corn starch. 4 Conclusion The functional properties of NPS (lower levels of retrogradation and syneresis and highest solubility when compared to native corn starch), which is a non-conventional source of starch, suggest it may have potential use in food systems. The acid hydrolysis (3.2 mol.L–1 HCl and 44 °C) of pinhão and corn starches caused gel thermo-reversibility, lower tendency to retrogradation of starch pastes, and higher solubility at an economically viable reaction time (6 hours). Furthermore, the APS showed a melting point (46 °C) close to that of the conventional fats (37-45 °C), which may indicate that the pinhão starch could be used as a fat substitute when the gel is prepared (5%, w/w dry basis, total weight 28 g) by heating at 95 °C for 30 minutes. However, this applicability is limited to Ciênc. Tecnol. Aliment., Campinas, 33(Supl. 1): 89-94, fev. 2013 frozen or refrigerated food since the acid hydrolysis reduced the tolerance of both starches to refrigerate storage (5 °C) and to the freeze-thaw cycles. Acknowledgements The authors thank Florencia Cladera-Olivera and Mauricio Seibel Luce for the critical reading of the manuscript and very helpful discussions and comments. References AGBOOLA, S. O.; AKIMBALA, J. O.; OGUNTIMEIN, G. B. Physicochemical and functional properties of low DS cassava starch acetates and citrates. Starch/Starke, v. 43, p. 62-66, 1991. http:// dx.doi.org/10.1002/star.19910430207 ALBRECHT, J. J.; NELSON, A. I.; STAINBERG, M. P. Characteristics of corn starch and starchs derivatives as affected by freeze storage and thawing. Food Technology, v. 14, p. 57-60, 1960. AMAYA-LLANO, S. L. et al. Isolation and partial characterization of banana starches. Journal of Agricultural and Food Chemistry, v. 47, p. 854-857, 1999. http://dx.doi.org/10.1021/jf980828t ATICHOKUDOMCHAI, N. et al. A study of some physicochemical properties of high-crystalline tapioca starch. Starch/Starke, v. 53, n. 11, p. 577-581, 2000. http://dx.doi.org/10.1002/1521379X(200111)53:11<577::AID-STAR577>3.0.CO;2-0 BELLO-PÉREZ, L. A. et al. Isolation and Characterization of Starch from Seeds of Araucaria brasiliensis: A novel Starch for Application in Food Industry. Starch/Starke, v. 58, p. 283-291, 2006. http:// dx.doi.org/10.1002/star.200500455 BEMILLER, J. N. Starch modification: Challenges and prospects. Starch/Starke, v. 49, p. 127-131, 1997. http://dx.doi.org/10.1002/ star.19970490402 CORDENUNSI, B. R. et al. Chemical composition and glycemic index of Brazilian pine (Araucaria angustifolia) seeds. Journal of Agricultural and Food Chemistry, v. 52, p. 3412-3416, 2004. PMid:15161207. http://dx.doi.org/10.1021/jf034814l FLECHE, G. Chemical modification and degradation of starch. New York: Dekker, 1985. 99 p. PMid:2419317. FLORES-GOROSQUERA, E. et al. Rendimiento del proceso de extracción del almidón a partir de frutos de plátano (Musa paradisiaca): Estudio en planta piloto. Acta Científica Venezolana, v. 55, n. 1, p. 86-90, 2004. PMid:15916169. FRANCO, C. M. L. et al. Culturas de Tuberosas amiláceas latino americanas-Propriedades gerais do amido. São Paulo: Fundação Cargill, 2002. 221 p. GIESE, J. Fat, oils and fat replacers. Food Technology, v. 50, n. 4, p. 77-83, 1996. HERMANSSON, A. M.; SVEGMARK, K. Developments in the understanding of starch functionality. Trends in Food Science and Technology, v. 7, p. 345-53, 1996. http://dx.doi.org/10.1016/ S0924-2244(96)10036-4 HOSENEY, R. C. Principles of cereal science and technology. St. Paul: American Association of Cereal Chemists, Inc., 1994. p. 52-54. LAWAL, O. S. Composition physicochemical properties and retrogradation characteristics of native, oxidized, acetylated and acid-thinned new cocoyam (Xanthosoma sagittifolium) starch. Food Chemistry, v. 87, p. 205-218, 2004. http://dx.doi. org/10.1016/j.foodchem.2003.11.013 93 E-19 International Journal of Biological Macromolecules 80 (2015) 557–565 Contents lists available at ScienceDirect International Journal of Biological Macromolecules journal homepage: www.elsevier.com/locate/ijbiomac Effect of citric acid concentration and hydrolysis time on physicochemical properties of sweet potato starches Ayenampudi Surendra Babu a , Ramanathan Parimalavalli a,∗ , Shalini Gaur Rudra b a b Department of Food Science and Nutrition, School of Professional Studies, Periyar University, Salem 636011, Tamil Nadu, India Department of Food Science and Post Harvest Technology, Indian Agriculture Research Institute, New Delhi 110012, India a r t i c l e i n f o Article history: Received 29 April 2015 Received in revised form 25 June 2015 Accepted 12 July 2015 Available online 15 July 2015 Keywords: DSC Fat replacer SEM Sweet potato starch XRD a b s t r a c t Physicochemical properties of citric acid treated sweet potato starches were investigated in the present study. Sweet potato starch was hydrolyzed using citric acid with different concentrations (1 and 5%) and time periods (1 and 11 h) at 45 ◦ C and was denoted as citric acid treated starch (CTS1 to CTS4) based on their experimental conditions. The recovery yield of acid treated starches was above 85%. The CTS4 sample displayed the highest amylose (around 31%) and water holding capacity its melting temperature was 47.66 ◦ C. The digestibility rate was slightly increased for 78.58% for the CTS3 and CTS4. The gel strength of acid modified starches ranged from 0.27 kg to 1.11 kg. RVA results of acid thinned starches confirmed a low viscosity profile. CTS3 starch illustrated lower enthalpy compared to all other modified starches. All starch samples exhibited a shear-thinning behavior. SEM analysis revealed that the extent of visible degradation was increased at higher hydrolysis time and acid concentration. The CTS3 satisfied the criteria required for starch to act as a fat mimetic. Overall results conveyed that the citric acid treatment of sweet potato starch with 5% acid concentration and 11 h period was an ideal condition for the preparation of a fat replacer. © 2015 Elsevier B.V. All rights reserved. 1. Introduction Today’s dietary concern is the consumption of huge quantity of fat and sugar. With the mounting incidence of diabetes and obesity, low calorie foods have acquired the huge esteem. In general, the best suitable approach in terms of fat reduction diets involves either the use of low-fat foods or fat substitutes or modifications such as trimming of fat from foods [1,2]. Fat Replacers consist of mixtures of lipid-originated fat substitutes, protein- or carbohydrate-originated fat mimetic, or their combinations [3]. Carbohydrate-based Replacers incorporate water into a gel type structure, resulting in a lubricant or flow properties similar to those of fats in food systems [2]. Even though a variety of fat replacers have been developed, there are unfortunately no ideal fat replacers which completely function like conventional fat [4]. Native starch can sometimes be used to replace fat [1]; however starch modified by acid or enzymatic hydrolysis, oxidation, Abbreviations: NS, native sweet potato starch; CTS, citric acid treated starch; HT, hydrolysis time; AC, acid concentration; DE, dextrose equivalent; WHC, water holding capacity; PV, peak viscosity; BD, break down; TV, trough viscosity; SB, setback; FV, final viscosity; Pt, pasting time; PT, pasting temperature; To, onset temperature; Tp, peak temperature; Tc, final temperatures; H, gelatinization enthalpy. ∗ Corresponding author. E-mail address: [email protected] (R. Parimalavalli). dextrinization, cross linking, or mono-substitution is more commonly used to achieve desired functional and sensory properties [1]. Generally, acid hydrolysis occurs more rapidly in amorphous regions than in crystalline region and the residue after prolonged acid hydrolysis consists of acid-resistant crystalline parts of amylopectin [5]. Thys et al. [6] investigated the functional properties of acid-thinned pinhao starch and it showed low syneresis, high solubility, thermo reversibility and melting point similar to fat. They concluded that the acid treatment was efficient in producing a potential fat substitute from pinhao starch. Amaya-Llano et al. [7] produced acid hydrolyzed jicama starch and used as a fat substitute in yoghurt. The addition of hydrolyzed jicama starch (2.03 g/100 g) as a fat substitute in the preparation of stirred yoghurt had good functional and sensorial properties. Ma et al. [8] reported that enzymatic hydrolyzed corn starch could be used as fat replacers. The hydrolyzed starch with fine particles was used to produce low fat mayonnaise and the result indicated that the 60% fat-reduced mayonnaise with fat replacers had similar sensory quality as compared with the high fat one. The following are the criteria for a starch based fat mimetic – (a) Starch should contain an amylose content of ∼20% [9]. (b) Starch ought to require a granule size of 2 m or in similar size to liquid micelle to act as fat mimetic [10]. (c) According to FDA [11] a starch-based fat mimetic is supposed to be partially or completely digestible. (d) Starch must possess a DE (dextrose equivalent) of http://dx.doi.org/10.1016/j.ijbiomac.2015.07.020 0141-8130/© 2015 Elsevier B.V. All rights reserved. E-20 560 A. Surendra Babu et al. / International Journal of Biological Macromolecules 80 (2015) 557–565 evaluating the effect of citric acid concentration (AC) and hydrolysis time (HT) on the response variables (Table 1). The data of physiochemical and functional properties of the citric acid treated starches were six replications. All data obtained were subjected to one way Analysis of Variance (ANOVA) or student t-test using SPSS program (Statistical Package for Social Science) version 14.0 (SPSS Inc., Illinois, USA). Comparison of means was performed using Tukey–Kramer HSD at P < 0.05. 4. Results and discussion 4.1. Physiochemical properties 4.1.1. Yield of isolated and acid treated sweet potato starches The yield of isolated sweet potato starch was 10.20%. The recovery yield of citric acid modified sweet potato starches was above 85% (Table 2) and it was in the range as reported by Dutta et al. [28] and Babu et al. [29]. With the increase in acid concentration, yield was reduced as starches might be hydrolyzed more rapidly at higher acid concentration. 4.1.2. Analysis of ash, protein, fat and total fiber The isolated native sweet potato starch had 0.26 ± 0.11% ash, 0.25 ± 0.14% protein, 0.07 ± 0.02% fat and 0.57 ± 0.10 total fiber. These values were consistent with the earlier report [30]. The isolated starch had minor protein and fat contents. 4.1.3. Dextrose equivalent (DE) DE value is an indication of extent of acid hydrolysis. The degree of citric acid hydrolysis of sweet potato starch was not severe as revealed by dextrose equivalent (DE) value. The DE value of acid-thinned sweet potato starches (Table 2) was ranged between 1.90 and 2.34% and it showed that the DE value was increased with increased acid concentration and hydrolysis time. Since CTS4 starch was treated for a greater hydrolysis conditions, it exhibited a higher DE value of 2.34% compared to their counterparts. Thys, Aires, Marczak, and Norena [6] reported a DE value of 6.5 for pinhao starch treated with HCl. Nevertheless DE values obtained in the present study was much lower as citric acid used for acid treatment was a weak organic acid, hence the degree of hydrolysis of these starches seems to be lower. Since the DE value of acid treated starches were within the range referred by National Starch Table 1 Hydrolysis conditions. Treatments HT (h) AC (%) NS CTS1 CTS2 CTS3 CTS4 – 1 1 11 11 – 1 5 1 5 NS, native starch; CTS1.CTS4, citric acid treated starches; HT, hydrolysis time; AC, acid concentration. and Chemical Corporation [12], indicates a potential applicability of the citric acid thinned sweet potato starch as a fat mimetic. 4.1.4. Apparent amylose The apparent amylose content of NS was 18.56%, which was on par with Tsakama, Mwangwela, Manani, and Mahungu [31]. Acid treated starches found to display a higher fraction of apparent amylose which was significant with NS. The highest apparent amylose (around 31%) content was noticed for CTS4 sample. This increased trend was in linear fashion with acid concentration and hydrolysis period. The removal of lipids from the starch samples by acid may result in higher value for amylose content. Increase in amylose might also be due to the formation of intramolecular and intermolecular linkages between residues of amylose, which increases the length of these chains and their capacity to form complexes with iodine, increasing the apparent amylose values. Another possible reason might be due to the de-polymerization of amylopectin fractions on continuous acid hydrolysis. High degree of acid hydrolysis led increased apparent amylose content of starch [32]. Starches with a higher linear fraction (amylose content) are able to bind strongly and orient water to endow with a sensation comparable to the rheology of fat in the oral cavity [33] hence CTS4 starch could mimic the functionality of fat when used as a fat replacer. Vanderveen and Glinsmann [9] suggested that starch should possess a 20% amylose to act as a fat replacer. 4.1.5. Moisture and dry matter Moisture content and dry matter of NS was 14.11% and 85.89%, respectively, whereas all acid thinned starches showed low moisture content and high dry matter. Hydrolysis time showed a noticeable effect on the moisture content. Increase in hydrolysis time would provide ample time for the starch to react with citric acid which results in increase in moisture content of starch. This pattern might be related to the reaction between the OH groups of glucose units of starch and the functional groups (OH) of citric acid used in this chemical modification, decreasing the possibility of reaction between OH of starch chains and the water molecules. Consequently, the probability of joining of water to this polymer would be reduced causing decrease in moisture content of modified starch thereby increase in dry matter [34]. A similar trend was observed by Omojola, Manu, and Thomas [35] during acid hydrolysis of cola starch. 4.1.6. Melting point, clear point and gel thermo-reversibility Melting point, clear point and thermo-reversibility of native and acid-thinned sweet potato starches are shown in Table 2. All the starches illustrated a perfect gel formation when gelatinized and stored under refrigeration at 4 ◦ C. Melting point of NS was observed at 69.33 ◦ C whilst the acid treated starches melted at a temperature lower than the native starch. The CTS4 starch had shown its melting temperature at 47.66 ◦ C, similar to the melting point of fats, which indicated that it would be used as a fat substitute. Amylopectin plays a major role in starch granule crystallinity and the presence of amylose indirectly lowers the Table 2 Physicochemical properties of native and acid-thinned sweet potato starches. S. No. Starch recovery yield (%) DE (%) NS CTS1 CTS2 CTS3 CTS4 100 92.93 89.84 91.33 85.05 ± ± ± ± ± – 1.90 ± 2.21 ± 2.04 ± 2.34 ± 0.00aA 1.10bB 1.24cB 0.80bB 2.56cC Apparent amylose (%) 0.08aA 0.05bA 0.03aA 0.05bB 18.56 24.78 29.59 30.27 31.04 ± ± ± ± ± 1.06aA 1.36bB 1.32cB 1.88bC 1.38cC Moisture (%) 14.11 6.62 11.00 11.30 11.83 ± ± ± ± ± 2.17aA 1.45bB 1.00aA 0.60aA 1.04aA Dry matter (%) 85.89 93.38 89.00 88.70 88.17 ± ± ± ± ± 0.80aA 3.07bB 1.00abB 1.47bAB 0.76abB Melting point (◦ C) 67.00 56.33 50.33 55.66 47.66 ± ± ± ± ± 2.64aA 2.04bB 2.65cB 1.86bB 1.94cC Clear point (◦ C) GTR In vitro digestibility (%) – 68.33 ± 61.00 ± 66.33 ± 58.33 ± No Yes Yes Yes Yes 63.27 72.59 70.59 76.22 78.54 0.57aA 1.52bA 1.52aB 2.88bB ± ± ± ± ± 4.27aA 11.67aA 5.78aA 7.18abA 7.40bB Mean values followed by different letters in the same column indicate significant difference (p < 0.05). Lowercase letters indicate significant difference in hydrolysis times while uppercase letters indicate significant difference in acid concentrations. DE, dextrose equivalent; GTR, gel thermo reversibility. E-21 A. Surendra Babu et al. / International Journal of Biological Macromolecules 80 (2015) 557–565 561 Table 3 Crystallinity, granule size and functional properties of native and acid-thinned sweet potato starches. Samples Crystallinity (%) NS CTS1 CTS2 CTS3 CTS4 35.33 34.26 38.80 40.50 35.55 ± ± ± ± ± 2.62aA 1.63aA 1.31aA 0.50bB 0.50aA Granule size (m) 8.61 8.08 8.67 8.00 8.66 ± ± ± ± ± 5.32aA 2.77aA 2.39aA 3.01aA 4.12aA Water holding capacity (%) 34.90 36.21 46.13 38.80 56.15 ± ± ± ± ± Emulsion activity (%) 3.31aA 1.17aA 3.12bB 3.48bB 4.20aA 54.44 66.45 65.71 66.30 68.62 ± ± ± ± ± 1.92aA 0.79bB 1.37cbB 1.72cbC 1.53bB Emulsion stability (%) 41.60 42.89 42.72 41.78 42.99 ± ± ± ± ± 1.75aA 2.06aA 1.62aA 1.28aA 4.05aA Mean values followed by different letters in the same row indicate significant difference (p < 0.05). Lowercase letters indicate significant difference in hydrolysis times while uppercase letters indicate significant difference in acid concentration. Table 4 Textural profile of native and acid thinned sweet potato starch. Samples Hardness (kg) NS CTS1 CTS2 CTS3 CTS4 1.64 1.11 0.56 0.28 0.27 ± ± ± ± ± 0.07aA 0.10bB 0.32cB 0.02bC 0.00bB Adhesiveness (kg/s) 0.05 0.01 0.00 0.08 0.02 ± ± ± ± ± 0.01aA 0.00bB 0.00bB 0.01bA 0.00aB Springiness 0.74 0.77 0.65 0.76 0.82 ± ± ± ± ± Cohesiveness 0.01aAB 0.11aA 0.04aB 0.02aA 0.07aA 0.49 0.71 0.49 0.49 0.61 ± ± ± ± ± 0.05aA 0.13aB 0.11aA 0.00aA 0.00bA Chewiness 0.40 0.39 0.16 0.10 0.13 ± ± ± ± ± 0.11aA 0.00aA 0.07bB 0.00bB 0.01bB Gumminess 0.54 0.63 0.23 0.14 0.16 ± ± ± ± ± 0.14aA 0.04aA 0.09bB 0.01bB 0.00bB Mean values followed by different letters in the same row indicate significant difference (p < 0.05). Lowercase letters indicate significant difference in hydrolysis times while uppercase letters indicate significant difference in acid concentrations. melting point of the starch granule [36]. Acid treatment of sweet potato starch might result in the formation of shorter amylopectin chains with less stable crystalline structure consequently facilitating a lower melting point and clear point [37]. Thys, Aires, Marczak, and Norena [6] noticed a melting point of 46 ◦ C for acid treated pinhao starch. All the treated starches had shown a clear point around 60 ◦ C however the clear point was not displayed by native starch even at 80 ◦ C and it might be above 80 ◦ C. Native starch resisted the gel thermo-reversibility. Conversely, acid-thinned sweet potato starches displayed gel thermo-reversibility which implies acid treatment of starch caused partial hydrolysis of starch chains, resulting in lower paste viscosity. However, when the paste cools down, acid-thinned starch chains tend to associate with each other more easily, forming a thermo-reversible gel [38]. Similar fashion of gel thermo-reversibility was registered in the previous study [6] for pinhao and corn starches. 4.1.7. In vitro digestibility Table 2 displays the in vitro digestibility of NS and CTS which was measured by glucoamylase. The in vitro digestibility of NS was about 63.27% and it was in the range with the previous report on sweet potato starch (28.3–67.2%) [38]. The digestibility rate of the CTS4 sample was significantly increased up to 78.54%. In view of the fact that acid hydrolysis preferentially attacks the amorphous area in the starch granule, the crystallites were decoupled from the amorphous parts consequently, unlocked amorphous regions would be more sensitive to the enzyme attack and prone to rapid hydrolysis on the external glucose residues of amylose or amylopectin. In the report of Shi et al. [39] HylonV starch, normal maize starch and waxy maize starch samples when subjected to acid treatment resulted in less resistant to ␣-amylase digestion. After acid hydrolysis of the starches, the amorphous structure of the starches was hydrolyzed, so that the density of the residual amorphous structure of the starches decreased. However, as the specific surface area increased, starches were easily reacted with the enzymes and as a result the hydrolysis rate of starch samples was greater than native starch. This higher digestibility of citric acid treated starches would be beneficial for its role as a fat replacer since FDA [11] recommended that a fat replacer to be partially or completely digestible. 5. Functional properties 5.1. Water holding capacity (WHC) WHC of NS was 34.90%. A significant difference was observed in the WHC among the starches (Table 3). The highest and lowest water holding capacity was detected in CTS4 starch and CTS1 starches respectively. WHC was directly proportional to the acid concentration and as well as hydrolysis time. High acid concentration probably increased the low molecular weight starch fraction with hydroxyl groups which may hold water molecules forming hydrogen bonds consequently increasing the WHC. This high water holding capacity of citric acid treated starch (CTS4) may find a significant role as a fat replacer. 5.2. Emulsion activity and emulsion stability Acid thinned starch, CTS4 exhibited a higher emulsion activity and emulsion stability compared to native starch (Table 3). Starch (CTS4) with higher amylose found to exhibit superior emulsion properties. The present study revealed that higher amount of linear amylose fraction would contribute to emulsion activity. The high amylose starch might function as the interface between oil and water during which linear amylose chains of starch granules were more favored in stabilizing the emulsion system than branched amylopectin chains. The linear amylose fractions could be capable of film formation that enhances the emulsion capacity and stability of the starch [40]. No significant difference in emulsion stability was noticed among the starches. 6. Texture analysis The texture profile analysis of NS and CTS samples is shown in Table 4. The gel strength of NS was 1.644 kg and acid modified starches ranged from 0.27 kg to 1.11 kg. The gel formed from NS was harder than CTS samples due to the degree of long chains in sweet potato native starch which contributed to its firmer gel. The lesser gel strength of the acid-thinned sweet potato starch might be attributed to a higher degree of short chains due to acid hydrolysis [41]. Wang and Wang [41] reported that gel strength (GS) of 0.30, 0.48 and 0.09 kg for acid thinned corn, potato and rice starches respectively. E-22 562 A. Surendra Babu et al. / International Journal of Biological Macromolecules 80 (2015) 557–565 Table 5 Pasting properties of native and acid thinned sweet potato starch. Sample Peak viscosity (cP) NS CTS1 CTS2 CTS3 CTS4 6338.00 6097.00 4812.00 4736.33 4655.33 ± ± ± ± ± 340.54 aA 128.31 aA 79.30bB 113.95bB 190.11bB Trough viscosity (cP) 3288.00 3027.00 1971.66 2248.66 1738.33 ± ± ± ± ± 189.59aA 62.69aA 55.94bB 84.31bB 144.36cB Break down (cP) 3050.00 3070.00 2840.33 2487.66 2917.00 ± ± ± ± ± 186.34aA 77.11 aA 83.18aA 34.50aA 53.45 aA Final viscosity (cP) 4290.66 4087.33 3028.66 3190.66 2683.66 ± ± ± ± ± 168.35aA 75.79 aA 83.57 bB 120.35 bB 189.95cB Set back (cP) 1002.66 1060.33 1057.00 942.00 945.33 ± ± ± ± ± Peak time (min) 24.68abA 48.67aA 29.05 bA 41.60aA 45.65aA 4.00 3.93 3.82 3.91 3.80 ± ± ± ± ± 0.07 aA 0.00 aA 0.04bB 0.03aA 0.07aAB Peak temperature (◦ C) 70.81 71.00 71.51 70.76 70.73 ± ± ± ± ± 0.49aA 0.82aA 0.49 aA 0.40aA 0.37aA Mean values followed by different letters in the same row indicate significant difference (p < 0.05). Lowercase letters indicate significant difference in hydrolysis times while uppercase letters indicate significant difference in acid concentrations. A similar significant decrease in the hardness of chick pea starch gel upon acid treatment was reported by Sodhi, Chang, Midha, and Kohyama [42]. Springiness represents the ability of a gel to recover its original shape/height after a deforming force is removed [43]. No significant change in the springiness was noticed due to hydrolysis time. Adhesiveness is the ability of the gel sample to become sticky [44]. It is a surface characteristic which depends on a combined effect of adhesive and cohesive forces, viscosity, and viscoelasticity of the sample [45]. Adhesiveness of NS and CTS was ranged between 0.00 g/s and 0.08 g/s. Starch with high amylose content was observed to have lower adhesiveness [46]. Cohesiveness is how well a sample withstands a second deformation related to how it behaved under the first deformation [47]. Cohesiveness indicates how good the sample retains its structure after the first compression. Cohesiveness of starch samples was ranged from 0.49 to 0.71. Gumminess is the product of hardness and cohesiveness, a characteristic of semi-solid foods which have a low degree of hardness and a high degree of cohesiveness [47]. NS displayed a higher chewiness compared to the CTS samples while CTS1 showed a higher gumminess than NS. The difference in textural properties of all sample gels was influenced by rigidity in gelatinized starch, amylose content as well as interaction between the dispensed and continuous phase of the gel, which in turn was dependent on the amylose and amylopectin structure [48]. 7. Pasting properties Table 5 reveals a significant influence of acid concentration and reaction time on RVA of the acid thinned sweet potato starch. RVA results of the acid thinned starches confirmed a low PV ranging from 4655.33 cP to 6097.00 cP compared to native starch (6338.00 cP). Results showed that the PV decreased with increase in acid concentration and reaction time. Similar fashion of change was reported in the literature [28,49]. CTS1 presented a viscosity profile higher than their counterparts, although substantially lower than the native starch. The lower PV of acid modified starches could be due to considerable breakdown of amorphous regions and the production of low molecular weight dextrins [50]. TV and BD values of CTS samples also displayed the same decreasing trend. The increased degree of amylose recrystallization by acid thinning might be due to the change in BD [51]. Acid thinned sweet potato starches displayed a lower FV ranging between 3028.66 and 4087.33 cP against 4290.66 cP for sweet potato native starch. Han, Campanella, Mix, and Hamaker [52] reported that acid hydrolysis resulted a considerable lyses of glycoside linkages of the long amylopectin chains, which apparently caused the fall in FV. SB is a measure of recrystallization of gelatinized starch. CTS1 and CTS2 registered a higher SB than NS indicating that these starches got a higher retrogradation tendency than NS. The low SB of the rest of the acid-thinned starches was likely due to in sufficient time for the starch molecules to rearrange themselves during the stipulated period [53]. Native starch took more time (4 min) to reach its PV than acid thinned starches. Hydrolysis time and acid concentration basically did not affect the PT of sweet potato starch in native form and acid modified form, however with the increase Table 6 Thermal properties of native and acid-treated sweet potato starch. Gelatinization temperature (◦ C) Sample To NS CTS1 CTS2 CTS3 CTS4 42.31 35.81 35.35 35.97 39.44 Tp ± ± ± ± ± 2.12aA 1.61bB 0.83bB 1.00bB 1.50aA 81.25 83.58 81.56 83.75 86.75 H (J/g) Tc ± ± ± ± ± 0.66 aA 2.50aA 1.77aA 0.03cA 0.25bB 116.12 120.10 119.43 116.77 122.69 ± ± ± ± ± 2.01 aA 2.85aA 2.06aAB 2.04aA 2.33bB 12.96 12.74 12.13 12.69 11.95 ± ± ± ± ± 0.05aA 0.03aA 0.80aA 1.29aA 1.31aA To, Tp and Tc stand for onset, peak, and conclusion temperatures respectively. H (J/g) indicates enthalpy. Mean values followed by different letters in the same row indicate significant difference (p < 0.05). Lowercase letters indicate significant difference in hydrolysis times while uppercase letters indicate significant difference in acid concentrations. in acid concentration and hydrolysis time, mild changes in pasting parameters were noticed. A similar decrease in pasting profile was observed upon acid treatment of corn by Singh, Sodhi, and Singh [50] in acid thinned sorghum starch. 8. Thermal analysis Thermal properties of starches determined by the DSC are represented in Table 6. Results showed variations in To, Tp, Tc temperatures and H among NS and CTS samples. NS had higher To of 42.31 ◦ C while citric acid treated starches showed a decreased trend. This was in agreement with the result reported for acid modified maize starch which showed a decreased To value [54]. The Tp and Tc of native starch were 81.25 ◦ C and 116.12 ◦ C respectively, conversely the acid modified starches displayed a higher Tp and Tc temperatures. However, this trend was more pronounced in case of CTS4. This specifies that a higher degree of hydrolysis might occur in CTS4 at amorphous region, thus resulting in an increase in relative crystallinity and subsequently an increase in the gelatinization temperature. Similar results were reported for acid modified potato starch [40] and sweet potato starch [55]. The H of NS was 12.96 J/g, on the other hand H of acid modified starches was lower than their counterpart. Enthalpy of the citric acid treated starches ranged between 11.95 and 12.74 J/g. CTS4 starch showed a lower enthalpy compared to all other modified starches. The decrease in H was the result of citrate substitution that altered the chain packing and generated more amorphous region [56]. H gives an overall measure of crystallinity and is an indicator of the loss of molecular order within the granule during gelatinization [57]. The increase in citric acid concentration and treatment time decreased the enthalpy that might be due to greater loss of ordered structure of starch 9. Rheological analysis Shear rate versus shear stress plot at 85 ◦ C was well fitted to the power law model (Eq. (1)) with determination coefficients (R2 ) ranging from 0.97 to 0.99, represented in Table 7. Native and acid modified starches displayed the values of flow behavior indexes (n) less than 1 indicating a shear-thinning behavior. NS showed a E-23 African Journal of Pure and Applied Chemistry Vol. 5(9), pp. 307-315, 9 September, 2011 Available online at http://www.academicjournals.org/AJPAC ISSN 1996 – 0840 ©2011 Academic Journals Full Length Research Paper Effect of acid hydrolysis on the physicochemical properties of cola starch Omojola M. O.1*, Manu N.1 and Thomas S. A.2 1 Raw Materials Research and Development Council PMB 232, Garki Abuja, Nigeria. Sheda Science and Technology Complex, Federal Ministry of Science and Technology, Sheda Abuja, Nigeria. 2 Accepted 22 July, 2011 Cola starch from Cola nitida (rubra) was isolated using 1% (w/v) sodium metabisulphite solution and was treated with 0.1 and 0.2 M HCl solution differently at 80 and 100°C, pH (6 to 7.9) and reaction time (30 min to 3 h). The physicochemical and functional properties of the hydrolyzed starch were studied. The hydrolysis reaction presented important changes in the pasting, thermal transition and morphology of the native starch. Reaction time, temperature and concentration of the acid were observed to influence the reactions. The acid modified starch has the following properties; swelling 6.13 to 7.21% solubility 14.83 to 16.65% and amylose content 17.28 to 21.69%, while the corresponding values for the native cola starch were swelling 8.85%, solubility 7.48% and amylose 24.76%. The rapid visco analysis (RVA) of the acid modified starch demonstrated low peak viscosity ranging from 52.71 to 197.22 as against 314.42 reported for the native starch. Breakdown viscosity and the setback values also exhibited the same decreasing trend; 30.24 to 73.17 and 10.18 to 34.91 respectively, as against that of native cola starch that has a breakdown and setback viscosity of 179.25 and 74.42 respectively. The observed trends are consistent with other modified starches that have found useful applications in pharmaceutical, food and confectionary industries. Key words: Cola, native starch, acid modified (thinned) starch, hydrolysis, composition. INTRODUCTION Native (unmodified) starches have different functional properties depending on the crop source and are considered a primary resource that can be processed into a range of starch products. The limited application of native starches is due to low shear resistance, thermal resistance, thermal decomposition and high tendency towards retro gradation, high syneresis, extreme processing conditions such as pH, temperature etc., (Cousidine, 1982). The limitations experienced from native starch may be overcome by various modifications, Jacobs and Delcour (1998). The basis of starch modification lies in the improvement of its functional properties by changing the physical and chemical properties of such native starch (Ortoefer, 1984). Starch modification which involves the alteration of the physical and chemical characteristics of the native starch can be used to improve its functional characteristic thereby tailoring it to specific applications (Hermansson and Svegmark, 1996). It is generally achieved through derivatization such as etherification, esterification, cross linking and grafting of starch, acid or enzymatic hydrolysis, oxidation or physical treatment of starch using heat or moisture. The modified starches generally show better paste clarity, better stability, increased resistance to retro gradation and increased freeze- thaw stability (Zheng et al., 1999). Recently, cola starch was isolated from Cola nitida (rubra spp) and characterized in our laboratory, (Omojola et al., 2010). Its physicochemical characterization showed high industrial potentials in the pharmaceutical, food and confectionary industries. The present study is to modify the starch through acid hydrolysis, evaluate its physicochemical properties, compared with that of the native starch and other acid thinned starches and suggest possible industrial applications. MATERIALS AND METHODS *Corresponding author. E-mail: [email protected]. C. nitida (rubra) were procured directly from the farm source in E-24 Omojola et al. 309 Plate 1. Photomicrograph of native cola starch. Plate 2. Photomicrograph of acid thinned cola starch (0.1 M HCl, 100°C 1 h). morphological alterations. This is similar to the reported thermal behavior of corn starch modified by acid treatment (Bennica et al., 2008). Acid hydrolysis The results of the acid hydrolysis of the native cola starch using 0.1 and 0.2 M HCl at 80, 100°C at different heating times from 30 min to 3 h are as shown in Tables 1 to 4 respectively. The results show that heating temperature, time and acid concentration affected the extent of hydrolysis or starch recovery. Any increase in the aforementioned parameter increases the extent of starch hydrolysis. This trend is similar to the results obtained in the acid modification of cassava starch (Ahmed et al., 2003) on prolonged treatment. Acid will attack both the amorphous and crystalline regions of the starch granule E-25 310 Afr. J. Pure. Appl. Chem. Plate 3. Photomicrograph of acid thinned cola starch (0.2 M HCl, 80°C 1h). Plate 4. Photomicrograph of acid thinned cola starch (0.2 M HCl, 100°C 1 h). to obtain water soluble molecules. Swelling and solubility The percent swelling and solubility profiles of the native cola and acid thinned starches are shown in Table 5. It can be seen that the swelling profile of acid thinned starch is lower than that of the native starch. This may be related to the changes in the surface characteristics of starch granules. At higher temperature, starch appears to lose its granular structure faster resulting in a low E-26 Omojola et al. 311 Plate 5. Photomicrograph of acid thinned cola starch (0.1 M HCl, 80°C 3 h). Plate 6. Photomicrograph of acid thinned cola starch (0.2 M HCl, 100°C 3 h). swelling capacity (Chang et al., 1995). Acid thinned starches are more soluble than native starches. The increase in solubility values may be due to shortening of the chain lengths of the starch, corresponding to the weakening of the hydrogen bonds (Osunsami et al., 1989), or due to the increasing hydroxyl groups (Aiyeleye E-27 312 Afr. J. Pure. Appl. Chem. Table 1. Acid hydrolysis of the native cola starch using 0.1 M HCl at 80°C. Parameter Extent of hydrolysis (%) Starch recovery (%) Final weight of starch (g) Moisture content (%) pH Final colour of starch 30 min 15.00± 0.05 85.00 ± 0.05 8.5 9.14± 0.01 7.2 Off- white Heating period 1h 2h 21 ± 0.00 24.99± 0.05 79.00± 0.00 75.01± 0.05 7.9 7.5 9.25± 0.00 9.45± 0.01 6.6 6.2 Off- white Off- white 3h 29.97±0.03 70.03±0.03 7.0 10.32±0.00 6.0 Off- white Table 2. Acid hydrolysis of the native cola starch using 0.1 M HCl at 100°C. Parameter Extent of hydrolysis (%) Starch recovery (%) Final weight of starch (g) Moisture content pH Final colour of starch 30 min 16.00± 0.00 84.00± 0.00 8.4 9.25± 0.01 7.5 Off- white Heating period 1h 2h 20.00±0.05 25.58±0.15 80.00±0.05 74.42±0.15 8.0 7.42 9.34± 0.01 9.66± 0.00 7.5 6.9 Off- white Off- white 3h 29.98±0.04 70.02±0.04 7.0 9.86± 0.02 6.2 Off- white Table 3. Acid hydrolysis of the native cola starch using 0.2 M HCl at 80°C. Parameter Extent of hydrolysis (%) Starch recovery (%) Final weight of starch (g) Moisture content pH Final colour of starch Heating period 30 min 20.00± 0.01 80.00± 0.01 8.0 9.18± 0.01 7.3 Off- white 1h 25.98± 0.04 74.04± 0.04 7.9 9.23± 0.00 7.3 Off- white 2h 30.00± 0.00 70.00± 0.00 7.5 9.44± 0.02 6.9 Off- white 3h 32.95± 0.15 67.05± 0.15 7.0 9.62± 0.02 6.4 Off- white Table 4. Acid hydrolysis of the native cola starch using 0.2 M HCl at 100°C. Parameter Extent of hydrolysis (%) Starch recovery (%) Final weight of starch (g) Moisture content pH Final colour of starch 30 min 21.02± 0.05 78.98± 0.05 7.9 9.19± 0.02 7.4 Off- white et al., 1983). It has also been reported that the high solubility of acid modified starch with increasing temperature may be due to the loss of granular structure and release of amylose fraction of the starch, as the amylose molecules are preferentially solubilized and leached from swollen granules (Stone et al., 1984). Heating period 1h 2h 25.00± 0.01 30.00± 0.00 75.00± 0.01 70.00± 0.00 7.5 7.0 9.23± 0.01 9.34± 0.02 7.3 6.8 Off- white Off- white 3h 35.00± 0.00 65.00± 0.00 6.5 9.45± 0.00 6.0 Off- white Amylose/ amylopectin contents of acid thinned starch The percent amylose/ amylopectin contents of acid thinned starch as presented in Table 6 shows the effect of reaction time on the amylase content of acid thinned E-28 Omojola et al. 313 Table 5. Percent swelling power and solubility of acid thinned cola starch. Starch properties Native cola starch 0.1 M HCl, 80°C, 1 h 0.1 M HCl, 80°C, 3 h 0.1M HCl, 100°C, 1 h 0.1M HCl, 100°C, 3 h % Swelling 8.85 7.21±0.02 6.47±0.00 7.05±0.02 6.13±0.01 % Solubility 7.48±0.02 14.83±0.02 15.98±0.00 15.00±0.01 16.65±0.02 Table 6. Percent amylose/amylopectin contents of acid thinned cola starch at different acid concentrations, reaction time and temperatures. Acid concentration 0.1 M HCl 0.1 M HCl 0.1 M HCl 0.1 M HCl 0.1 M HCl 0.1 M HCl 0.1 M HCl 0.1 M HCl 0.2 M HCl 0.2 M HCl 0.2 M HCl 0.2 M HCl 0.2 M HCl 0.2 M HCl 0.2 M HCl 0.2 M HCl Temperature of heating (°C) 80 80 80 80 100 100 100 100 80 80 80 80 100 100 100 100 Heating period 30 min 1h 2h 3h 30 min 1h 2h 3h 30 min 1h 2h 3h 30 min 1h 2h 3h % Amylose 21.69±0.03 20.18±0.04 20.09±0.00 19.66±0.02 21.56± 0.03 20.15±0.00 20.10±0.00 19.35±0.01 21.46±0.01 20.04±0.00 20.03±0.04 17.82±0.00 20.88±0.02 19.99±0.00 18.14± 0.02 17.28±0.03 % Amylopectin 78.31±0.03 79.82±0.04 79.91±0.00 80.34±0.02 78.44 ±0.03 79.85±0.00 79.90±0.00 80.65±0.01 78.54±0.01 79.96±0.00 79.97±0.04 82.18±0.00 79.12±0.02 80.01±0.00 81.86±0.02 82.72± 0.03 N/B: The percent amylose/ amylopectin of native cola starch = 24.76/ 75.24. starch. The amylose content of acid modified starch is lower than the unmodified one. This is in line with earlier reported work on tapioca and corn starches (Napporn et al., 2001; Ya-June et al., 2003). The decreasing trend in the amylose content of the acid thinned starch as the reaction time increases corresponds to different concentrations of the HCl used and for the different heating temperatures. 30.24 to 73.17 as compared to 179.25 for the native one. The value decreases as the reaction time increases. The final viscosity showed a reduction from 209.58 for the native starch to 21.18 to 157.93 for the acid thinned starch. It also shows a decreasing trend as the reaction time increases. The setback viscosity which is lower than that of native starch suggests that such starch may find application in the food industry. Pasting properties Gelatinization properties Table 7 shows the comparative RVA of the acid thinned and native cola starches. The peak viscosity of the acid thinned ranged from 52.71 to 197.32 and is lower than that of native cola starch of 314.42. The table also shows that the peak viscosity decreases as the reaction time increases. Similar trends have earlier been reported for tapioca starch (Napporn et al., 2001). The low peak viscosity suggests that it can be used in forming gels in gums and jellies. The breakdown viscosity ranged from The data obtained for the DSC thermo gram of the acid thinned starch is as shown in Table 8. The results showed variations in the onset, peak and gelatinization temperatures, of both the acid thinned and native cola starches. Native starch has onset temperature (To) as 85.5°C and a peak temperature (Tp) of 318.1°C, while the 0.1 M HCI, 80°C, 3 h hydrolyzed cola starch, recorded (TO) of 37.7°C, with a (Tp) of 252.9°C and 0.2 M HCI, 100°C, 1 h E-29 314 Afr. J. Pure. Appl. Chem. Table 7. Pasting properties (RVA) of acid thinned cola starch at different acid concentrations, heating periods and temperatures. Profile of the starch Native cola starch 0.1 M HCl, 80°C 1 h 0.1 M HCl, 80°C 2 h 0.1 M HCl, 80°C 3 h 0.1 M HCl, 100°C 1 h 0.1M HCl, 100°C 2 h 0.1 M HCl, 100°C 3 h 0.2 M HCl, 80°C 30 min 0.2 M HCl, 80°C 1 h 0.2 M HCl, 80°C 2 h 0.2 M HCl, 80°C 3 h 0.2 M HCl, 100°C 30 min 0.2 M HCl, 100°C 1 h 0.2 M HCl, 100°C 2 h 0.2 M HCl, 100°C 3 h Peak visc. Trough visc. Breakdown visc. Final visc. Set back Peak time 314. 42 115.51±0.00 114.16±0.01 60.50±0.00 82.23±0.00 62.84±0.03 61.91±0.02 197.32±0.03 82.31±0.03 79.84±0.02 56.82±0.03 182.83±0.00 85.63±0.03 74.81±0.03 52.71±0.03 135.17 69.08±0.00 69.58±0.03 30.09±0.01 33.91±0.02 16.69±0.02 31.67±0.00 124.16±0.00 19.72±0.00 33.67±0.00 21.40±0.00 116.34±0.00 19.97±0.00 30.11±0.01 21±0.00 179.25 46.42±0.00 44.58±0.04 30.42±0.01 48.33±0.02 46.15±0.04 30.24±0.02 73.17±0.02 62.60±0.02 46.17±0.01 35.41±0.01 65.67±0.00 65.67±0.00 44.70±0.01 31.53±0.04 209.58 96.40±0.02 91.65±0.04 43.51±0.00 48.18±0.03 46.91±0.02 41.33±0.00 157.93±0.02 49.93±0.02 46.78±0.02 34.34±0.02 151.27±0.03 30.32±0.03 23.91±0.00 21.18±0.04 74.42 25.48±0.04 23.93±0.03 13.44±0.00 13.66±0.01 10.67±0.01 10.24±0.03 33.74±0.02 10.18±0.02 12.93±0.02 12.71±0.02 34.91±0.01 10.33±0.01 10.03±0.03 9.71±0.02 4.80 4.27 4.27 4.20 4.20 4.13 4.13 4.27 4.13 4.22 4.27 4.27 4.20 4.13 4.13 Pasting temp. 74.50 85.60 85.75 85.80 84.65 84.70 84.70 84.88 85.85 85.90 85.90 84.05 84.80 85.80 85.80 Table 8. Gelatinization properties of acid thinned cola starch. Starch properties Native starch 0.1 M HCl, 3 h 0.2 M HCl, 1 h Glass transition temp. (°C) 301 37.7 42.4 Peak temp. Tp (°C) 321.1 252.9 226.9 Endset temp. (°C) H (Tc-To) 340 66.5 66.7 39 28.8 24.3 Pasting temp. (°C) 74 65.80 64.70 syrups, jellies and gum products. They can also be employed as stabilizers in sausages and dressings. hydrolyzed cola starch has (TO) of 42.4°C and (Tp) of 226.9°C. The gelatinization temperature of the native cola starch was recorded as 74.00°C, while that of the acid treated starch ranged between 64.70 to 65.80°C. The lower gelatinization properties observed for the acid thinned starches may be due to the weakening of the hydrogen bond during acid hydrolysis. The formation of new chemical group in starch granule and depolymerization of starch granules result in the lowering of transition temperature. The second author wishes to thank the management of the Raw Materials Research and Development Council, Abuja. Nigeria for the provision of the research grant to execute this work. Conclusion REFERENCES Acid hydrolysis of cola starch affected its physicochemical, thermal and morphological properties. The extent of modification depends on the acid concentration, reaction time and temperature. Acid hydrolysis increased starch solubility and lowers its swelling capacity. It also resulted in lower peak, set back and breakdown viscosities than was reported for the native starch. The lower peak viscosity which may be related to increasing crystallinity suggests that such starch could be employed as tablet filler in the pharmaceutical industry. Acid thinned starches may also be utilized in the candy industry in the manufacture of Ahmed AS, Igbo UE, Igwe CC (2003). Evaluation of the physicochemical properties of acid thinned cassava starch. Nigerian Food J., 23: 85-88. Aiyeleye FB, Akingbala JO, Oguntimein GD (1983). Chemical factors affecting acetylation of cassava starch. Starch, 45: 443-445 Bennica C, Demiate IM, Lacerda LG ,Carvalho F ,Lonashiro M, Schnittzer E (2008); Thermal behavior of corn starch granules modified by acid treatment. Ecletica Quimica., 23(3): 13-18. Chang YL, Shao YY, Tseng KH (1995). Gelation mechanism and rheological properties of rice starch. Cereal Chem., 74(4): 339. Cousidine DM (1982). Foods and food production encyclopedia. John Wiley Inc., p. 142. Hermansson AM, Svegmark K (1996). Developments in the understanding of starch functionality. Trends Food Sci. Technol., 7: 354-363. ACKNOWLEDGEMENT E-30 International Food Research Journal 24(Suppl): 265-273 (December 2017) Journal homepage: http://www.ifrj.upm.edu.my Mini review A review on acid and enzymatic hydrolyses of sago starch Azmi, A.S., Malek, M.I.A. and *Puad, N.I.M. Department of Biotechnology Engineering, Kulliyyah of Engineering, International Islamic University Malaysia, P.O. Box 10, 50728 Kuala Lumpur, Malaysia Article history Abstract Received: 17 May 2017 Received in revised form: 10 June 2017 Accepted: 10 July 2017 This paper reviews reported studies on the hydrolysis of starch especially sago via acid and enzyme. The review begins with overview of sago palm and the starch industry, followed by process of extracting the starch from sago pith. Physicochemical properties of sago starch were tabulated for better understanding of hydrolysis process. Factors or process condition influencing hydrolysis process is discussed based on results from previous researches. Advantages and disadvantages of each hydrolysis is also discussed. Generally, there are very few researches dedicated on sago starch as compared to other starches. It can be concluded that, enzyme hydrolysis gives higher yield at milder process conditions. However, the reaction rate of enzyme hydrolysis is still low compared to acid hydrolysis. Keywords Sago starch Acid hydrolysis Enzymatic hydrolysis Introduction In Malaysia, sago palm (Metroxylon spp.) is widely planted especially in Sarawak and Johor. Sago industry is so well established here in the Eastern state of Malaysia which lead to their contribution towards economic revenue with 25,000-40,000 tons of sago products being produced annually (Singhal et al., 2008). The starch is processed for direct food consumption, pharmaceutical product and fermentable sugar for others different products through bioconversion. One of the processes involved is hydrolysis. Hence, the objective of this paper is to review previous studies on sago starch, specifically starch hydrolysis. This paper focuses on the two techniques to hydrolyze starch, which are acid and enzymatic hydrolyses. Both techniques have their own advantages and disadvantages that need to be considered before choosing the suitable method for treating the starch for further applications. Sago palm Sago palm (Metroxylon sagu) is a type of plant native to countries in tropical southeastern Asia such as Malaysia, Indonesia, Papua New Guinea and Thailand. Since ancient time, it acts as an important source of carbohydrate to the native population. Locally known as ‘rumbia’, Melanau communities in Sarawak consume starch obtained from sago palm as their staple food source (Mohamad Naim et al., 2016). *Corresponding author. Email: [email protected] © All Rights Reserved Many scientists consider sago palm as the ‘starch crop of the 21st century’ (Singhal et al., 2008). This is due to many characteristics that makes it a quite remarkable plant. Firstly, sago is an extremely resistant plant that able to survive in swampy, acidic peat soil (Chew et al., 1999). Furthermore, the palm is immune to floods, drought, fire and strong winds. Sago forest also acts as an excellent carbon sink which helps in mitigating the greenhouse effect and global warming arising from the release of carbon dioxide into the atmosphere. Second special characteristic is that it does not need replanting since the plant itself continually produce suckers which in turn grow into adult palm. This consequently eliminates the need for recurring expensive establishment costs after every harvest of the adult palm. Thirdly, among starchproducing crops, sago palm gives the highest yield of starch with potentially up to 25 tons of starch per hectare per year. In term of per unit area, the yield could be about 3 to 4 times higher than that of rice, corn, or wheat, and about 17 times higher than that of cassava (Karim et al., 2008). In short, in this age of concern for the environment and economy, sago is the crop par excellence for sustainable agriculture and profitability. Sago starch industry Sago palm is an important commercial tropical crop in Malaysia. Sarawak is the state in Malaysia where the trees are planted in abundance with 67,957 hectares of land (Mohamad Naim et al., 2016) E-31 Azmi et al./IFRJ 24(Suppl): S265-S273 269 Table 3 Summary of studies on acid hydrolysis of starch NR = Not Reported the factors are; type of starch, starch or substrate concentration and viscosity, enzyme concentration, temperature, pH, reaction duration, agitation rate, and starch pretreatment. Types of starch influence the degree of hydrolysis and the reducing sugar produced. Uthumporn et al. (2010) studied on hydrolysis of granular starch at sub-gelatinization temperature of 35oC for 24 h using mixture of alpha-amylase and glucoamylase. They observed that sago has the highest resistance to enzymatic degradation compared to corn, mung bean and cassava starches. This is due to the presence of pores on starch surfaces which are likely to become center of enzymatic attack. Uthumporn et al. (2010) study was also consistent with other studies from O’Brien and Wang (2008), Wang et al. (1996), Zhang and Oates (1999), Regy and Padmaja (2013). Substrate concentration and viscosity is related to one another and therefore they are discussed together here. Firstly, substrate concentration is the amount of substrate per total solution while viscosity is a property of fluid that indicates resistance to flow. Generally, increasing the concentration of a dissolved or dispersed substance will lead to increase in viscosity. Starch is a source for thickening agent or thickener which functions to increase the viscosity of a liquid without substantially changing its other properties. Wee et al. (2011) reported the effect of high substrate concentration towards the yield of reducing sugar after hydrolysis using glucoamylase. It was concluded that as the substrate concentration keep increasing, yield of reducing sugar will decrease due to high viscosity of starch solution that resulted in the poor mixing of samples. Furthermore, Uribe and Sampedro (2003) stated that solvent viscosity results in friction against proteins in solution, and this should result in decreased motion, as well as inhibiting catalysis. Enzyme concentration is the amount of enzyme used per total solvent, and it is an important parameter to look at. If enzyme concentration is too low, reaction will take place at a slower rate and resulted in the low yield. If enzyme concentration is too high, it can lead to underutilized of the enzyme and this situation should be avoided since commercial enzymes are currently expensive. From the same study as before, Wee et al. (2011) observed that a higher yield of reducing sugar was obtained when enzyme concentration increases but further increase in concentration did not influence the yield. Hence, it is clear that providing a proper amount of enzyme for the reaction is very important. On the other hand, most of the studies used multiple enzymes which reflect the intended purpose of the reaction (i.e. liquefaction or saccharification). Hence, α-amylase and glucoamylase were the main enzymes involved in most studies (Table 4). Interestingly, pullulanase, a debranching enzyme, has been utilized in some studies which serve to prevent the reverse reaction of glucose condensation catalyzed by glucoamylase (Findrik et al., 2010). However, study conducted by Wee et al. (2011) showed that by using only single enzyme which was glucoamylase, an approximately 60% of sugar yield from sago starch can be obtained. Temperature is one of the crucial factors in the enzymatic hydrolysis. This is because many enzymes are adversely affected at the high temperatures and are completely destroyed at 100°C. Besides, each enzyme has its own optimum temperature for it to work properly and become active. The activity of an enzyme is decreased when the temperature of E-32 270 Azmi et al./IFRJ 24(Suppl): S265-S273 Table 4 Summary of studies on enzymatic hydrolysis. the reaction differed from its optimum temperature. Amenaghawon et al. (2016) conducted a study of enzymatic hydrolysis towards cocoyam starch and found that the rate of hydrolysis was faster at a higher temperature. Reaction at 80°C for 10 min resulted in 72.06 g/L of reducing sugar while 75.22 g/L of reducing sugar was obtained when reacted at temperature of 90°C for the same duration. Hence, this means increasing the temperature of a system will increase the number of collisions of enzyme and E-33 Azmi et al./IFRJ 24(Suppl): S265-S273 substrate, thus increasing the rate of reaction. The pH of a solution can have several effects towards the structure and activity of enzymes. As explained by Khanna (2010), the pH can have an effect of the state of ionization of acidic or basic amino acids. If the state of ionization of amino acids in a protein is altered then the ionic bonds that help to determine the 3D shape of the protein can be changed. This can lead to altered protein recognition or an enzyme might become inactive. Furthermore, changes in pH may not only affect the shape of an enzyme but it may also change the shape or charge properties of the substrate so that either the substrate cannot bind to the active site or it cannot undergo catalysis. In general, enzyme has its own optimum pH value and the value is not the same for each enzyme. Reaction duration and agitation rate are related to each other. Since enzyme action involves the collision between the substrate and enzyme, agitation will result in faster time needed to complete the reaction. Mussatto et al. (2008) studied on the effect of agitation speed, enzyme loading and substrate concentration towards enzymatic hydrolysis of cellulose. Later, it was found that agitation speed did not significantly affect glucose yield. From here, it can be concluded that the amount of glucose yield does not depend so much on the agitation rate since substrate concentration is the limiting factor. However, agitation provides a proper mixing of the reactants which ultimately shorten the time needed to complete the reaction. Sago starch have a low digestibility and they are resistant to both microbial and enzyme digestions. Granule size could be one of the factors that contribute to this phenomenon and hence the need of pretreatment comes to the fore. Pretreatment such as annealing process (O’Brien and Wang, 2008), autoclaving and microwave in water or dilute acid (Sunarti et al., 2012), or mechanical pretreatment such as crusher, viz juice mixer, homogenizer and high speed planetary mill (Kumakura and Kaetsu, 1983) influence the efficiency of enzymatic hydrolysis process (Table 4) and plays important role in preparing the starch for enzyme attack and degradation. Outlook Sago palm is an important carbohydrate source especially to tropical southeastern Asia. The trunk is processed through several processes to obtain the starch. The starch extraction and washing processes resulted with solid and liquid residues which also rich of starch for further process. The knowledge of physicochemical properties of the starch is important 271 to effectively process the starch. In general, sago starch granule has bigger size and is resistance to enzyme degradation compared to other types of starch. Furthermore the presence of pores on the granule surface of other types of starch is susceptible to enzyme attack. Thus, pretreatment sometimes is required prior to hydrolysis process especially when using enzyme. Starch hydrolysis can be accomplished using acid or enzyme. Not many researches are devoted to sago starch as compared to other starches. Despite of that, several physical factors have been studied to maximize the production yield. Acid hydrolysis is a simple method, easily available and cheap. However, few drawbacks such as relatively low yield, high process temperature and formation of undesirable by­products shifted the option to enzyme. Enzyme is highly selective and reaction specific produce less unwanted byproduct, give higher yield to glucose at milder process which requires less energy. However, it gives low reaction rate and high sugar monomers which create difficulty for separation and yet to be economical production. Acknowledgment Sincere thanks to International Islamic University Malaysia Research Initiative Grant (RIGS16-0890253). References Abd-Aziz, S. 2002. Sago starch and its utilisation. Journal of Bioscience and Bioengineering 94(6): 526-529. Abdorreza, M., Robal, M., Cheng, L., Tajul, A. and Karim, A. 2012. Physicochemical, thermal, and rheological properties of acid-hydrolyzed sago (Metroxylon sagu) starch. LWT-Food Science and Technology 46(1): 135-141. Ahmad, F.B., Williams, P.A., Doublier, J.-L., Durand, S. and Buleon, A. 1999. Physico-chemical characterisation of sago starch. Carbohydrate Polymers 38(4): 361-370. Albani, J. R. 2007. Principles and applications of fluorescence spectroscopy. Oxford, UK: Blackwell. Amenaghawon, N., Osagie, E. and Ogbeide, S. 2016. Optimisation of Combined Acid and Enzymatic Hydrolysis of Cocoyam Starch to Produce Fermentable Hydrolysate. Pertanika Journal of Science and Technology 24(1): 123-136. Awg-Adeni, D., Abd-Aziz, S., Bujang, K. and Hassan, M. 2010. Bioconversion of sago residue into value added products. African Journal of Biotechnology 9(14): 2016-2021. Awg-Adeni, D.S., Bujang, K., Hassan, M.A. and AbdAziz, S. 2012. Recovery of glucose from residual starch of sago hampas for bioethanol production. BioMed Research International 2013. http://dx.doi. E-34 Food Rev. Int., 16(3), 369–392 (2000) Downloaded by [University of Arizona] at 04:17 14 January 2013 ACID-TREATED STARCHES R. HOOVER Department of Biochemistry Memorial University of Newfoundland St. John’s, Newfoundland Canada A1B 3X9 ABSTRACT Acids such as HCl and H2SO4 cause scission of the glucosidic linkages, thereby altering the structure and properties of the native starch. The amorphous regions of the starch granule are more susceptible to acid hydrolysis than the crystalline regions. This review summarizes the current knowledge on: (1) the extent of acid hydrolysis of starches from different botanical origins; (2) the changes in molar mass, crystallinity, viscosity, gel rigidity and gelatinization transition temperatures on acid hydrolysis; (3) the effect of annealing, heat–moisture treatment, high pressure, and amylose-complexed lipids on the rate and extent of acid hydrolysis and; (4) the mechanism of acid hydrolysis in an alcoholic media. INTRODUCTION Acid hydrolysis has been used to modify starch granule structure and produce ‘‘soluble starch’’ for many years (1). Nägeli (2) reported the treatment of native potato starch in water with 15% H2SO4 for 30 days at room temperature. He obtained an acid-resistant fraction that was readily soluble in hot water. This fraction has come to be known as Nägeli amylodextrin and has been shown to be a mixture of lowmolecular-weight, linear, and branched dextrins, with an average degree of polymerization (DP) of 25–30. Subsequently, Lintner (3) described an acid modification of native potato starch in which granules were treated in an aqueous suspension with 7.5% (w/v) HCl for 7 days at room temperature. The product was a high-molecular369 Copyright 2000 by Marcel Dekker, Inc. www.dekker.com E-35 ORDER REPRINTS Downloaded by [University of Arizona] at 04:17 14 January 2013 370 HOOVER weight starch, which formed a clear solution in hot water. This is used as an indicator in iodometric titration and for enzyme analysis. In industry, acid-modified starches (maize, waxy maize, wheat, and cassava) are prepared by treating a starch slurry (40%) with dilute HCl or H2SO4 at 25–55°C for various time periods. The conditions used during acid hydrolysis are influenced by the ratio of the cold to hot paste viscosity and by the required gel texture. When the desired viscosity or fluidity is attained, the starch slurry is neutralized, and the granules are recovered by washing, centrifugation, and drying. Industrial uses of acid hydrolyzed starches are as follows: (a) as a premodification step for the production of cationic and amphoteric starches (4); (b) as a warp sizing agent to increase yarn strength and abrasion resistance in the weaving operation (4); (c) for preparation of starch gum candies (4); (d) for manufacture of gypsum board for dry wall construction (4); and (e) for paper and paperboard manufacture (4). Recently, Chun et al. (5) have shown that rice amylodextrins prepared by hydrolyzing rice starch in acidic (4% HCl) alcohol (70%) solutions at 78–80°C was readily solubilized with warm water (50°C). Emulsions prepared by replacing a portion of the oil (used in the formulation of a mayonnaise-type emulsion) with rice amylodextrin, exhibited small and uniform droplets and displayed high viscosity and stability. This suggests that amylodextrins could be used as fat replacers (5). This article summarizes the current knowledge on the susceptibility of native, annealed, heat–moisture treated, lipid-complexed, and pressure-treated starches (from different botanical origin) towards hydrolysis by acid, and on the structure and properties of the residue left after acid hydrolysis. The last section deals with the role of alcohols in acid hydrolysis. MECHANISM OF ACID HYDROLYSIS In acid hydrolysis, the hydroxonium ion (H3O⫹ ) carries out an electrophilic attack on the oxygen atom of the α(1 → 4) glycosidic bond (Fig. 1a). In the next step, the electrons in one of the carbon–oxygen bonds move onto the oxygen atom (Fig. 1b) to generate an unstable, high-energy carbocation intermediate (Fig. 1c). The carbocation intermediate is a Lewis acid, so it subsequently reacts with water (Fig. 1d), a Lewis base, leading to regeneration of a hydroxyl group (Fig. 1e). SOLUBILIZATION PATTERNS OF STARCHES The solubilization profiles of some cereal, tuber, and legume starches are presented in Figures 2a and 2b. All starches exhibit a two-stage hydrolysis pattern. A relatively fast hydrolysis rate during the first 8 days followed by a slower rate between 7 and 12 days has been reported for corn, waxy corn, high amylose corn, wheat, potato, E-36 ORDER REPRINTS 371 Downloaded by [University of Arizona] at 04:17 14 January 2013 ACID-TREATED STARCHES Figure 1. Mechanism of acid hydrolysis of starch. oat, rice, waxy rice, smooth pea, lentil, wrinkled pea, adzuki bean, mung bean, and red kidney bean (6–15). When the hydrolysis data (Fig. 2a) is plotted as log10 (100/ 100–x) vs. time (days), the two-stage process is clearly evident (Fig. 2c). The faster stage corresponds to the hydrolysis of the more amorphous parts of the starch granule. During the second stage, the crystalline material is slowly degraded (6, 16). Evidence to suggest a preferential attack on amorphous domains within the granule comes from transmission electron microscopy observations of acid hydrolyzed E-37 COURSE BOOK OF CHEMISTRY 2 (BIOCHEMISTRY) Department of Biochemistry Benha University, Agriculture College PRACTICAL E-38 PRACTICAL BIOCHEMISTRY Course name Practical Biochemistry Teacher in charge Ahmed Mahmoud Hassan Mohamed Department/ College Biochemistry / Agriculture Contact Ahmed Mahmoud / [email protected] Course link at the University Course overview: In two or three constructive paragraphs mention the importance and the necessity of this course Biochemistry can be defined as the science concerned with the chemical basis of life. The cell is the structural unit of living system, thus biochemistry can also be described as the science concerned with the chemical constituents of living cells and with the reactions and processes they undergo. By this definition, biochemistry encompasses large areas of cell biology, of molecular biology, and of molecular genetics. Course objective: in two or three paragraphs mention the aims of the course and the main points students should have learned by the end of the course. We learn the students the general and specific tests for determine the normal subjects of biochemistry also the abnormal one. That related with diseases. E-39 Method: • Add 2 drops of the α-naphthol solution (5% in ethanol, prepare fresh) to 2 ml of test solution in a test tube. • Carefully, pour about 1 ml of conc. H2SO4 down the side of the tube so as to form two layers. • Carefully observe any colour change at the junction of the two liquids. • Repeat the test, using water instead of the carbohydrate solution. 2. Fehling’s Test: This forms the reduction test of carbohydrates. Fehling’s solution contains blue alkaline cupric hydroxide solution, heated with reducing sugars gets reduced to yellow or red cuprous oxide and is precipitated. Hence, formation of the yellow or brownish-red colored precipitate helps in the detection of reducing sugars in the test solution. Preparation of Fehling's solution A: Dissolve 35g of Cu2SO4.7H2O in water and make up to 500ml Preparation of Fehling's solution B: Dissolve 120 g of KOH and 173 g of Sod. Pot. Tartarate (Rochelle salt) in water and make up to 500 ml Fehling’s reagent: Equal volumes of Fehling A and Feling B are mixed to form a deep blue solution. Note: If you do not have sodium potassium tartarate, it can prepared using tartaric acid as described below. Method: • Mix equal volumes of Fehling's solution A and B. • Add 5 drops of the test solution (glucose, fructose, and sucrose solution) to the mixed Fehling's solution and boil. Results Glucose solution Orange-brown color is appeared. Fructose solution Orange-brown color is appeared. Sucrose solution No change. Discussion: Fehling's tests for aldehydes are used extensively in carbohydrate chemistry. A positive result is indicated by the formation of a brick red precipitate. Like other aldehydes, aldoses are easily oxidized to yield carboxylic acids. Cupric ion complexed with tartrate ion is reduced to cuprous oxide. The cupric ion (Cu++) is complexed with the tartarate ion. Contact with an aldehyde group reduces it to a cuprous ion, which the E-40 precipitated as orange-brown Cu2O. The sucrose does not react with Fehling's reagent. Sucrose is a disaccharide of glucose and fructose. Most disaccharides are reducing sugars, sucrose is a notable exception, for it is a non-reducing sugar. The anomeric carbon of glucose is involved in the glucose- fructose bond and hence is not free to form the aldehyde in solution. On the other hand, glucose, a reducing sugar, reacts with Fehling's reagent to form an orange to red precipitate. Fehling's reagent is commonly used for reducing sugars but is known to be not specific for aldehydes. For example, fructose gives a positive test with Fehling's solution too, because fructose is converted to glucose and mannose under alkaline conditions. The conversion can be explained by the keto-enol tautomerism. The reduction of Fehling solution using fructose is not only to be attributed to the fact that the ketose is isomerized into an aldose. The treatment of fructose with alkali - e.g. Fehling solution - causes even decompostion of the carbon chain. More products with reducing capability are formed. Note: Fehling's test takes advantage of the ready reactivity of aldehydes by using the weak oxidizing agent cupric ion (Cu2+) in alkaline solution. In addition to the copper ion, Fehling's solution contains tartrate ion as a complexing agent to keep the copper ion in solution. Without the tartrate ions, cupric hydroxide would precipitate from the basic solution. The tartrate ion is unable to complex cuprous ion Cu+, so the reduction of Cu2+ to Cu+ by reducing sugars results in the formation of an orange to red precipitate of Cu2O. Copper-tartrate-complex CuSO4 + NaOH Cu(OH)2 + Na2SO4 Cu(OH)2 + HO-CH-COONa O-CH-COONa + 2H2O HO-CH-COOK Cu O-CH-COOK R-CHO + Cu++ Cu+ + OH- 2OH- + Cu++ + HO-CH-COONa HO-CH-COOK R-COOH + Cu+ CuOH W.∆B. Cu2O Red ppt E-41 LEMBAR ASISTENSI DIPERIKSA KETERANGAN NO TANGGAL 1. 8-03-2021 P0 oleh asisten 2. 11-03-2021 P1 oleh asisten 3. 12-03-2021 P2 oleh asisten 4. 14-03-2021 ACC oleh asisten TANDA TANGAN