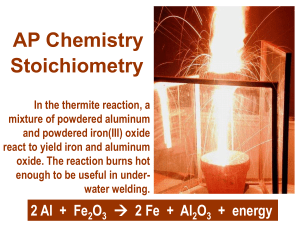
LESSON PLAN School : SMAN 9 SURABAYA Subjects : Chemistry Class / Semester: X / Even Main Material : Stoichiometry Time Allocation : 40 minutes A. Core Competencies IC 1 : Living up to and practicing the teachings of the religion they hold. IC 2 : Live and practice honest behavior, discipline, responsibility, care (mutual cooperation, cooperation, tolerance, peace), polite, responsive and proactive, and show attitude as part of the solution to various problems in interacting effectively with the social environment and nature and in placing themselves as a reflection of the nation in the world association. IC 3 : Understanding, applying, and analyzing factual, conceptual, procedural and metacognitive knowledge based on curiosity about science, technology, art, culture, and humanities with human, national, state, and civilization insights related to the cause of phenomena and events, and apply procedural knowledge to specific fields of study according to their talents and interests to solve problems. IC 4 : Cultivate, reason, and present in the realm of concrete and abstract domains related to the development of what they learn in school independently, act effectively and creatively, and be able to use methods according to scientific principles. B. Basic Competence and Competency Achievement Indicators Basic Competency Indicator Basic Competence Indicator 3.10 Apply basic laws chemistry, the 3.10.3 Determine the limiting reagents in a concept of relative molecular mass, reaction chemical equation, mole concept, and levels of substances to complete chemical calculation. C. Learning Objectives 1. Students can determine the limiting reagents when given an example of reaction. D. Learning Materials 1. The concepts of moles with limiting reagents. E. Learning Methods Learning Model: Direct Instruction Methods: Interactive lectures, questions and answers, discussions, and assignments F. Learning Media Media : • Worksheets / Student worksheets (LKPD) • PowerPoint slides (ppt) • Internet Tools / Materials : • Laptop • LCD • Stationery G. Learning Resources Erfan Priyambodo, dkk. 2016. Buku Siswa Kimia untuk SMA/MA Kelas X. Klaten: PT Intan Pariwara. Ernavita, dkk. 2007. Kimia SMA/MA Kelas X. Jakarta : PT Tunas Melati Internet H. Learning Steps The second meeting Learning Materials : The concepts of moles with limiting reagents. 1st Meeting (2 x 45 minutes) Direct Instruction Model Preliminary activities Teacher : Time 10 minute Giving greetings, appointing class leaders to lead the prayers, and checking s the absence of students. Encourage students to start the lesson. Asking students readiness to learn. Apperception (at the beginning of the chapter) : The teacher presents a phenomena drug reactions in our body. You know that drug in certain doses will break down into active substances and residual substances. Active substances will be used by the body to treat diseases, the remaining substances will be removed aas impurities. Stools in the form of urine, sweat, and faces. Why does it happen? What kind of reagent will run out first? We will learn it at this meeting. Core Activities 20 Learning minute model s syntax of Learning Activity Direct Instruction Clarify goals and establish The teacher conveys the learning objectives of the material to be discussed, namely: set 1. Students can determine the limiting reagents Learning objectives are displayed on the third slide of PowerPoint. Demonstrate • The teacher explains the procedure for determine the limiting knowledge or reagents displayed on PPT slides 4 - 6 1st Meeting (2 x 45 minutes) Direct Instruction Model skill • Students pay attention to the material delivered by the teacher. (observe) • After listening to the delivery of material from the teacher, students are asked to ask questions about things that are not understood from what was observed or questions to get additional information about what was observed. (asking question) Provide guided practice Check for explained. (try) Questions presented at LKPD page 1-3 The teacher guides the students through each step The teacher checks the student's work individually (around), understandin to make sure the student is working on the questions given by g and feedback The teacher gives exercises about the material that has been the teacher. Students are asked to write answers to questions that have been given on the LKPD page 3. Students and teachers check the answers of students written on the board, then the teacher straightens out if there are answers that are still wrong and give praise if the students' answers are correct (communicating) Provide estended practice and The teacher gives homework (homework) regarding the material that has been discussed. Questions from the homework are presented on LKPD page 4 transfer Note: During learning takes place, the teacher observes the attitude of students in learning, namely attitude: curiosity. Time 1st Meeting (2 x 45 minutes) Direct Instruction Model Time Closing Activity 5 Student : minute s Ask about unclear material. Teacher : Provide conclusions from the material that has been submitted. Inform students that at the next meeting an experiment will be conducted on review and practice questions for final examination. Remind students to do homework well and collect it at the next meeting. End the learning activities with prayer and greetings. I.Assessment 1. Assessment technique a. Knowledge Competency Assessment Written test Essay 2. Instrument Attached Knowing Headmaster of SMAN 9 SURABAYA Hj.RA.Roosdiantini, S.Pd,. M.Pd. NIP Surabaya, 15th May 2020 College Student in Chemistry UNESA Wahyu Ismi Zakiyah STUDENT WORKSHEET Name :…………………………………………………… Class :…………………………………………………… No :……………………………………………………. 1. Read the phenomena below! The formation of carbon monoxide in the vehicle exhaust. Perfect combustion of fuel forms water vapor and carbon dioxide. However, incomplete combustion produces water vapor and carbon monoxide. Why does it happen? Give the reason! ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… …………………………………………………………………… 2. Determine the question below based on the steps for determaining limiting reagent A total of 22 grams of C3H8 were reacted with 48 grams. C3H8 + O2 → CO2 + H2O Determine the substance which is a barrier and the mass of the remaining substance! a. To determine the limiting reagents, you must find the relative molecular masses and moles of C3H8 and O2. ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ……………….………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… …………. b. After the relative molecular and mole masses of C3H8 and O2 have been approved, you must equalize the reaction equation as follows : C3H8 + 5O2 → 3CO2 + 4H2O The limiting reagents can be determined by the following equation : ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ……………….………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… …………………………. c. In the reaction above, the remaining substance is C3H8, so you must find the remaining mass of C3H8. Before searching for the remaining mass of C3H8, we must know how many moles of C3H8 are left. ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………... ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… …………………………………………………………………………………….. 3. Determine the question below based on the steps in number 2! In a closed container, 20 grams of methane (CH4) are burned with 64 grams of oxygen (O2) producing carbon dioxide and water vapor according to the reaction below. It is known that Mr methane = 16, Ar oxygen = 16, and Mr H2O = 18. CH4 (g) + O2 → CO2 + 2H2O Determine the limiting reagent and the mass of the remaining substance! ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………... ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………... ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………... ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… HOMEWORK The equation for the reduction of iron ore in a furnace is as follows : Fe2O3 + 3CO → 2Fe + 3CO2 How many kilograms of iron (Molar mass 56 g/mole) can be produced if 7 kg Fe2O3 (Molar mass 160 g/mole) is reacted with 3 kg CO (molar mass 28 g/mol) according to the above reaction. How many kilograms of excess reagent after the reaction stops? ………………………………………………………………………………………… ………………………………………………………………………………………… ………………………………………………………………………………………… ……………….………………………………………………………………………… ………………………………………………………………………………………… GOOD LUCK