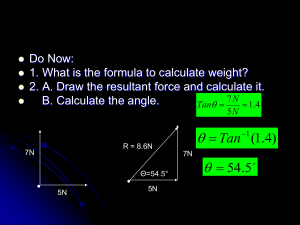
Pengaruh pH pada laju reaksi Misalkan hidrogen dibebaskan oleh reaksi 2H+ + 2e- H2 Konsentrasi ion hidrogen akan mempengaruhi laju reaksi As the hydrogen ion concentration is increased (i.e. the solution made more acid), so the rate of the reaction increases Similarly the potential will influence the reaction - the more negative the potential the faster the reaction Effect of pH and potential on rate of hydrogen evolution Slower Potential Faster pH Effect of pH on reaction rate On platinum no metal dissolution will occur, but to balance the charge a reaction which creates electrons must occur If the solution contains dissolved hydrogen, the reverse of the hydrogen evolution reaction can occur: H2 2H+ + 2e- Effect of pH on reaction rate H2 2H+ + 2e- This reaction will go faster in alkaline solution (since H+ will be removed by H+ + OH- H2O) This reaction will go faster at more positive potentials (because electrons will be removed from metal) Effect of pH and potential on rate of hydrogen oxidation Oxidation Faster Reduction Slower Potential Oxidation Slower Reduction Rates equal Faster Electrochemical Equilibrium pH Thermodynamic Equilibrium 2H+ + 2e- H2 The potential at which it occurs for a given solution composition is known as the equilibrium potential. The concentrations of reactants controls the rates of the forward and reverse reactions and hence the equilibrium potential Potential The Pourbaix (E-pH) Diagram 2.0 1.6 1.2 0.8 0.4 0.0 -0.4 -0.8 -1.2 -1.6 2H2O = O2 + 4H+ + 4eEquilibrium potential as pH increases Ofalls is stable 2 2H+ + 2e- = H2 Equilibrium potential falls as H2O is stable pH increases H2 is stable pH = - log [H+] 0 7 14 Pourbaix Diagram for Zinc Potential Equilibrium for 2.0 Zn(OH)2 + 2OH ZnO22- + 2H2O 1.6 Equilibrium for 1.2 2+ + 2OH- Zn(OH) Zn 2 0.8 Zn(OH) stable 0.4 ZnO22solid Equilibrium for 0.0 Zn2+ Equilibrium stable for stable in - Zn + 2OH Zn(OH) + 2e 2 -0.4 Zn Zn2+ + 2e- solution in for solution Equilibrium -0.8 2Zn + 4OH ZnO2 + 2H2O + 2e-1.2 Zn metal stable -1.6 0 7 14 2 2 Corrosion 2.0 1.6 1.2 0.8 0.4 0.0 -0.4 -0.8 -1.2 -1.6 possible with oxygen reduction Corrosion Corrosion is possible, but likely possible with to be stifled by solid hydrogen Zn(OH) Corrosion corrosion product stable evolution ZnO22CorrosionCorrosion requires solid is 2+ Zn stable stable in strong oxidising thermodynamically solution in solution agent impossible Passivity Potential Pourbaix Diagram for Zinc Corrosion Immunity Zn metal stable 0 7 14 Potential Pourbaix Diagram for Gold 2.0 1.6 C 1.2 0.8 0.4 0.0 -0.4 -0.8 -1.2 -1.6 0 Passivity C corrode Gold Gold metalcan’t stable with oxygen reduction Immunity or hydrogen evolution 7 14 Pourbaix diagram for Copper Will copper corrode in acid? Potential Will copper corrode in neutral waters? CuO22- stable in soln. 2.0 No - hydrogen 1.6 evolution only Cu oxides 1.2 occurs below the stable 0.8 Cu2+ stable potential for copper 0.4 corrosion in solution Usually it will0.0 just passivate,-0.4 but corrosion can-0.8 occur Cu metal stable in slightly acid -1.2 solutions -1.6 0 7 14 Pourbaix Diagram for Iron Will iron corrode in acid? 2.0 1.6 Yes - there is a 1.2 reasonably wide Fe3+ 0.8 range of potentials 0.4 Fe oxides where hydrogen Yes - although iron can stable can be evolved and 0.0 form an oxide in neutral 2+ iron dissolved Fe stable -0.4 solution, it tends not to -0.8 form directly on the No - iron forms a solid Fe metal stable -1.2 metal, as oxide the potential at all potentials, is too low, therefore is and-1.6 will itpassivate 7 14 not protective. 0 Potential WillWill ironiron corrode corrode in in alkaline neutral waters? solution? Potential Pourbaix diagram for Aluminium 1.2 0.8 0.4 0.0 -0.4 -0.8 -1.2 -1.6 -2.0 -2.4 Al3+ Al2O3 AlO2- Al 0 7 14 Limitations of Pourbaix Diagrams Tell us what can happen, not necessarily what will happen No information on rate of reaction Can only be plotted for pure metals and simple solutions, not for alloys