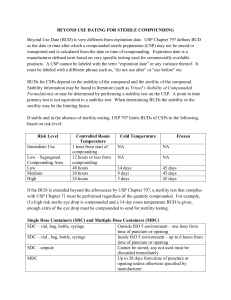
What is Water for Injection (WFI) ? 1. Introduction Water for Injection (WFI) is a prime example of how water is essential to life in the medical field. WFI is a highly purified, sterile medium that is essential for safely administering medications, whereas tap water supports plants and makes our morning coffee. We’ll use WFI frequently in this blog to show its significance, discuss how it’s different from other waters, and show how it’s made in accordance with the highest international standards. 2. WFI (Water for Injection): What Is It? Water that has been sterilized, purified, and packaged especially for parenteral (injectable) use is referred to as WFI in pharmaceutical and clinical contexts. Among WFI’s salient features are: ● Sterility: There are no observable living microorganisms in WFI. ● Endotoxin Control: To prevent hazardous pyrogenic reactions when WFI comes into contact with blood or tissue, the endotoxin level in WFI must be less than 0.25 Endotoxin Units (EU) per milliliter. ● Chemical Purity: Usually less than 500 parts per billion (ppb), WFI has a very low Total Organic Carbon (TOC). ● Microbial Limit: WFI must have no more than 10 Colony Forming Units (CFU) of aerobic bacteria per 100 mL, per the US Pharmacopeia (USP). This blog’s emphasis on WFI highlights how patient safety can be directly impacted by any compromise in WFI quality. 3. Comparing WFI with Other Pharmaceutical Waters Although phrases like Water for Injection (WFI), Sterile Water for Injection, and Bacteriostatic Water for Injection are frequently used synonymously, they have different meanings: 1. Water for Injection (WFI) ● The base grade is extremely purified using validated membrane techniques or distillation. ● Sterilization frequently takes place during drug manufacturing, so WFI might not be sterile right away. 2. Injectable Sterile Water (SWFI) ● WFI that has been further sterilized (autoclaving or 0.2 µm filtration, for example). ● Aseptically packaged to maintain sterility until use. 3. Bacteriostatic WFI ● WFI that contains benzyl alcohol and other antimicrobial preservatives. ● Allows for several uses from a single vial, but because of the potential for preservative toxicity, it is not appropriate for large‑volume or neonatal applications. The main product in each of these categories is still WFI, with each variation being made to meet particular manufacturing or clinical requirements. 4. The Significance of WFI Sterility and Purity WFI is a lifeline, and when we talk about it, we’re talking about more than just water. Any impurity in WFI could have detrimental effects: ● Infections: Bacteria can enter the bloodstream directly from non‑sterile WFI. ● Pyrogenic Shock: Fever, chills, or hypotension can be brought on by endotoxins in inadequate WFI. ● Drug Degradation: When organic pollutants in WFI interact with active pharmaceutical ingredients (APIs), the potency of the drug is diminished. To safeguard patient health, WFI’s sterility and purity requirements are therefore non‑negotiable. 5. WFI’s Main Applications in Healthcare WFI is essential to healthcare workers for a number of crucial tasks: ● Reconstitution of Drugs Antibiotics, vaccines, and biologics are among the many drugs that come in powder form. They are diluted or dissolved by WFI into injectable solutions. ● Flushing of IV Lines To avoid mixing incompatible medications, WFI flushes intravenous lines and catheters both before and after medication administration. ● Cleaning of Equipment To ensure aseptic conditions and remove residues, WFI is used to rinse fill needles, vials, stoppers, and processing equipment. ● Applications in the Lab WFI is used in diagnostic tests and cell culture media preparation where contaminants could distort the results. It is easier to understand why WFI production is strictly regulated when one considers that every application requires the same degree of WFI quality. 6. WFI’s Strict Production Pharmacopeial‑grade WFI is achieved by a number of carefully regulated steps: 1. Prior to Treatment ● Softening & Dechlorination: Preserves downstream membranes by eliminating minerals that cause scaling and leftover disinfectants. ● Activated Carbon Filtration: By absorbing organic pollutants and disinfectant byproducts, activated carbon filtration works. 2. First Purification ● Reverse Osmosis (RO): More than 99 percent of dissolved ions, bacteria, and organics are eliminated by reverse osmosis. ● Ultrafiltration (UF): Gets rid of high‑molecular‑weight contaminants and endotoxins. 3. Last‑Minute Polishing ● Distillation (Vapor Compression or Multi‑Effect): Removes endotoxins and volatile pollutants. ● UV Oxidation: Water is disinfected and trace organics are broken down by UV oxidation. 4. Aseptic Filling and Sterilization ● Membrane Filtration (0.2 µm) or autoclaving in a clean room. ● WFI is packaged in Grade A/B aseptic suites to maintain sterility. 5. Control of Quality ● Microbial count, TOC, conductivity, particulates, and endotoxin (LAL assay) testing. Every one of these steps guarantees that the finished WFI satisfies—or surpasses—pharmacopeial requirements, allowing it to be safely applied directly to human tissues. 7. International Standards and Adherence to Regulations WFI manufacturers adhere to rules set forth by: ● United States Pharmacopeia (USP) ● European Pharmacopeia (EP) ● Japanese Pharmacopeia (JP) ● World Health Organization (WHO) Although high‑grade membrane techniques may be allowed by the EP as an alternative to distillation, all compendia stress: ● Endotoxin Limits: ≤ 0.25 EU/mL ● Microbial Bioburden: ≥ 10 CFU/100 mL ● TOC Limits: < 500 ppb Routine regulatory inspections and in‑process sampling confirm that WFI production stays within these stringent parameters. 8. New Developments Improving WFI Manufacturing The need for efficiency and safety is what is driving the ongoing evolution of the WFI landscape: ● Continuous Purification Systems Reduce your energy footprint by integrating membrane and distillation processes to generate WFI around‑the‑clock. ● Monitoring in Real Time Conductivity, TOC, and bacterial counts are monitored by inline sensors, which enable prompt remedial action in the event that WFI quality deviates. ● Quality‑by‑Design (QbD) Statistical risk assessments identify crucial control points, moving the emphasis from end‑product testing to process validation. These developments simplify compliance with changing regulatory requirements and strengthen WFI quality. 9. Collaborating with WFI Professionals Choosing the correct WFI partner is crucial, regardless of whether you manage a hospital pharmacy or a pharmaceutical plant. Seek out suppliers who provide: ● Thorough WFI Testing Particulate analysis, TOC, conductivity, LAL endotoxin assays, and microbial cultures. ● Validation of Water Systems Installation Qualification (IQ), Operational Qualification (OQ), Performance Qualification (PQ), and continuous observation. ● Technical Assistance Proficiency with distillation and RO/membrane technologies, as well as familiarity with USP, EP, and WHO standards. ● Complete Turnkey Solutions End‑to‑end services include regulatory documentation, system design, installation, and maintenance. Working with professionals guarantees that your WFI systems continuously provide water that satisfies the highest standards. 10. Conclusion In conclusion, Water for Injection (WFI) is much more than just ultra‑pure water. It is essential to both pharmaceutical quality and patient safety. Healthcare professionals and pharmaceutical companies can depend on WFI to secure each injection, infusion, and laboratory procedure by being aware of the subtleties of WFI production, requiring stringent testing, and collaborating with reliable experts. After all, every drop of this exceptional water is vital to life.