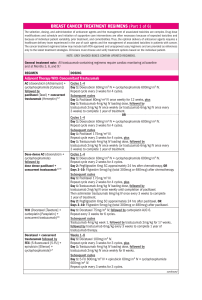
OBSTETRICS AND GYNECOLOGY ADVANCES BREAST SURGERY INDICATIONS AND TECHNIQUES No part of this digital document may be reproduced, stored in a retrieval system or transmitted in any form or by any means. The publisher has taken reasonable care in the preparation of this digital document, but makes no expressed or implied warranty of any kind and assumes no responsibility for any errors or omissions. No liability is assumed for incidental or consequential damages in connection with or arising out of information contained herein. This digital document is sold with the clear understanding that the publisher is not engaged in rendering legal, medical or any other professional services. OBSTETRICS AND GYNECOLOGY ADVANCES Additional books in this series can be found on Nova’s website under the Series tab. Additional e-books in this series can be found on Nova’s website under the e-book tab. OBSTETRICS AND GYNECOLOGY ADVANCES BREAST SURGERY INDICATIONS AND TECHNIQUES CIRO COMPARETTO AND FRANCO BORRUTO Copyright © 2018 by Nova Science Publishers, Inc. All rights reserved. No part of this book may be reproduced, stored in a retrieval system or transmitted in any form or by any means: electronic, electrostatic, magnetic, tape, mechanical photocopying, recording or otherwise without the written permission of the Publisher. We have partnered with Copyright Clearance Center to make it easy for you to obtain permissions to reuse content from this publication. Simply navigate to this publication’s page on Nova’s website and locate the “Get Permission” button below the title description. This button is linked directly to the title’s permission page on copyright.com. Alternatively, you can visit copyright.com and search by title, ISBN, or ISSN. For further questions about using the service on copyright.com, please contact: Copyright Clearance Center Phone: +1-(978) 750-8400 Fax: +1-(978) 750-4470 E-mail: [email protected]. NOTICE TO THE READER The Publisher has taken reasonable care in the preparation of this book, but makes no expressed or implied warranty of any kind and assumes no responsibility for any errors or omissions. No liability is assumed for incidental or consequential damages in connection with or arising out of information contained in this book. The Publisher shall not be liable for any special, consequential, or exemplary damages resulting, in whole or in part, from the readers’ use of, or reliance upon, this material. Any parts of this book based on government reports are so indicated and copyright is claimed for those parts to the extent applicable to compilations of such works. Independent verification should be sought for any data, advice or recommendations contained in this book. In addition, no responsibility is assumed by the publisher for any injury and/or damage to persons or property arising from any methods, products, instructions, ideas or otherwise contained in this publication. This publication is designed to provide accurate and authoritative information with regard to the subject matter covered herein. It is sold with the clear understanding that the Publisher is not engaged in rendering legal or any other professional services. If legal or any other expert assistance is required, the services of a competent person should be sought. FROM A DECLARATION OF PARTICIPANTS JOINTLY ADOPTED BY A COMMITTEE OF THE AMERICAN BAR ASSOCIATION AND A COMMITTEE OF PUBLISHERS. Additional color graphics may be available in the e-book version of this book. Library of Congress Cataloging-in-Publication Data ISBN: H%RRN Published by Nova Science Publishers, Inc. † New York Contents Preface vii Introduction xiii Chapter I Historical Overview 1 Chapter II Breast Biopsy 17 Chapter III Benign Breast Diseases 31 Chapter IV Breast Cancer 55 Chapter V Imaging-Guided Breast Surgery 85 Chapter VI Breast Lymphadenectomy 97 Chapter VII Endoscopic Breast Surgery 117 Chapter VIII Prophylactic Breast Surgery 123 Chapter IX Breast Reconstruction 137 Chapter X Aesthetic Breast Surgery 157 Chapter XI Breast Surgery Complications 181 Chapter XII Anesthesiological Issues 201 Chapter XIII Conclusion 207 References 209 About the Authors 275 Index 277 Preface Breast surgery is the surgical treatment of breast diseases. Its fields of application are particularly breast cancer therapy and the plastic-reconstructive time after it. Oncological surgery is one of the most important stages of breast cancer therapy. The history of breast interventions, known since ancient times, has been marked by several moments, coincident with the evolution of medical knowledges and with some important discoveries in this field. This allowed, after thousands of years, to arrive, by the end of the last century, to a type of intervention that, in the utmost respect for physical integrity, offers women a concrete chance of healing. From the oncological point of view, breast lymphatic structure is of particular importance, following that of the venous system, since it is well known that metastatic cancer spread mainly follows this pathway. In the early 1900s, breast cancer was believed to be a largely district-related disease that only in the advanced stages becomes systemic for the peripheral diffusion of metastases. In this period of time, William Stewart Halsted in 1894 published the results of a radical mastectomy intervention consisting of the removal en bloc of the breast, the large and small pectoral muscle, and the lymph nodes of the omolateral axillary excavation. This technique, albeit very demolishing, at a time when the disease was only revealed following clinical evidence, appeared rational and appropriate to ensure complete removal of the tumor and its diffusion paths before it had time to extend to the whole organism. In the last few decades, instead, thanks to the great progresses made in the field of instrumental diagnostics, the hypothesis that cancer is a systemic disease from the time of its inception, because it is very soon capable of producing micrometastases, has been growing. Consequently, in the presence of an already widespread illness, it is no longer meaningful to perform only a single district operation, moreover severely mutilating: it appears justified, instead, an operation that only removes the tumor locally but is associated and integrated with appropriate adjuvant therapies (chemotherapy, viii Ciro Comparetto and Franco Borruto hormone-therapy, immune-therapy, radiotherapy, etc.) that can clean systemic metastases. It has been also reinforced the notion that early diagnosis is essential because it allows to remove small tumors that are therefore able to produce more limited metastases than the bigger ones. In oncological surgery, and especially in breast cancer, the choice of intervention is subordinated to the stage at which the disease is diagnosed. Until some time ago, the diagnosis was almost exclusively clinical, based on the symptoms and, above all, on clearly visible signs at breast examination and palpation. These signs, punctually listed in breast cancer books, are: breast swelling, orange-peeling or stingy or ulcerated skin, nipple deviation or retraction, hemorrhage from galactofor ducts, etc., and they represent the obvious manifestation with which the disease, just because in an advanced stage, gives a sign of itself. These are, fortunately, increasingly rarer situations where the only surgical indication is a very aggressive operation that, although associated with medical therapies, often fails to change a severe prognosis. Today, however, the concept of preclinical diagnosis as the possibility to reveal the tumor before it becomes apparent with visible manifestations has been stated. In these cases, not only it is possible to have little or no demolitive surgery, but the survival and healing percentages significantly increase. This has come thanks to various reasons: 1) information and screening campaigns: targeted information campaigns have contributed to widening the participation of women in breast cancer screening. Important study groups against cancer, as the National Operational Force against Breast Cancer (FONCAM), have prepared appropriate protocols that, by identifying “target populations” (women over 40 years of age or with risk factors such as nulliparity, obesity, premature menarche, etc.) foresee a series of investigations that consist of breast examination, various instrumental investigations, etc.; 2) progress of instrumental diagnostics: mammographic examination has been usefully accompanied by ultrasound and the advent of increasingly sophisticated equipments allows to reveal tumors of a few millimeters (mm) and thus in the very beginning phase; and 3) diagnostic protocols: fundamental in the elaboration of the various therapeutic programs, they include a number of required steps. They begin with the common clinical and instrumental investigations that, in doubtful or positive cases, are followed by further examinations: cytological [on cells taken by fine-needle aspiration (FNA)] or histological (on fragments of tissue taken by biopsy). These samples, sometimes of a few mm and taken by the internal glands, can be purchased because they are ultrasonically or radiographically “guided” by sophisticated equipments designed for this purpose, such as the Mammotome [Devicor Medical Products, Inc., Cincinnati, OH, United States (USA)] or the Vacora system (Bard Biopsy Systems, Tempe, AZ, USA). By “classification” is meant the identification of predetermined categories in which cancer may be inserted. One of the best known and followed is the clinical (made at the time of diagnosis or at certain post-therapeutic moments) or the pathological (made at the time of surgical Preface ix intervention as it appears to the operator and especially to the pathologist who performs the microscopic exams extemporaneously) Tumor-Node-Metastasis (TNM) classification. It provides a large number of classes characterized by different values of: 1) T, that distinguishes primitive tumor whose size and nature is expressed by the value that accompanies it (X – 0 – IS – 1mic, 1a, 1b, 1c, 1d – 2 – 3 – 4a, 4b, 4c, 4d): TX or T0 indicate a non-definable or undetectable tumor, T1c a tumor of size up to 2 centimeters (cm), to progressively arrive to T4b, tumor of any size but already adherent to the skin in which it causes an inflection or orange peel, and to T4d, indicating inflammatory carcinoma; 2) N, indicating whether and to what extent breast lymph nodes are affected by the tumor: also the values that accompany N are different (X – 0 – 1a – 1b1, 1b2, 1b3, 1b4 – 2 – 3) and go from N0, absence of metastasis, to N3, when there is metastatic involvement of the lymph nodes belonging to the omolateral internal breast chain; and 3) M, that refers to the presence of any metastases: MX indicates that it is impossible to ascertain the presence of distant metastases, M0 excludes them, and M1 locates them in distant organs. Some examples: 1) T1cN0M0 pathological indicates a tumor of the size of 1-2 cm without metastatic involvement of local lymph nodes and without metastases in distant organs; 2) T4bN2M0 indicates a cancer already adhering to the skin with metastases in axillary lymph nodes (ALN) that are fixed and without peripheral metastases; and 3) T1aN0M1 indicates a tumor less than 0.5 cm without local lymphatic involvement but with metastases in distant organs. This big number of classes and subclasses makes it easy to type a cancer. This is useful because it allows to accurately integrate it into the most appropriate therapeutic protocol for that “type” of cancer because it uniquely and universally identifies it, and because it allows healthcare providers and other structures to monitor its evolution. Practical reasons and easy-tounderstand requirements have led to simplify the TNM system by choosing the most homogeneous classes and incorporating them into only four groups or stages, including some subcategories: 1) Stage 0: TisN0M0; 2) Stage I: T1N0M0; 3) Stage IIA: T0N1M0 or T1N1M0 or T2N0M0; 4) Stage IIB: T2N1M0 or T3N0M0; 5) Stage IIIA: T0N2M0 or T1N2M0 or T2N2M0 or T3N*M0 (* N1 or N2); 6) Stage IIIB: T4N*M0 (* with any N state) or T*N3M0 (* indicates any T state); and 7) Stage IV: T*N*M1 (* with any T and any N state). Staging allows the setting of the most suitable therapy, and an objective statistical evaluation of prognostic evolution. Breast cancer has been studied for many decades and the great mass of data collected has made it possible to produce reliable estimates of the outcome of the affected patients. This disease, the first cause of death from malignancy in women, when treated in the preclinical phase, has a very high survival rate at five and ten years, up to 95-98%. In contrast, at Stage IV, this percentage dramatically drops below 5%. But staging has also other advantages: 1) it avoids a difficult decision, to choose an intervention that may be more or less invasive and mutilating, to the empathic relationship between surgeon and patient; 2) it represents an x Ciro Comparetto and Franco Borruto objective and acceptable discussion basis for the patient who understandably tends to reject a mutilating intervention; and 3) it makes the therapeutic choice for that particular situation univocal, regardless of the surgeon, the structure, and the country where surgery is performed, with the exception, of course, of the operator’s professionalism and the quality of the service that can be varied. Tumor classification and staging, along with other general evaluations, allow the elaboration of therapeutic protocols indicating the most suitable type of intervention and possible adjuvant and/or neoadjuvant therapies to be associated. Current views on breast cancer surgical therapy generally suggest that tumors initially revealed, and therefore small, can be treated with conservative interventions, leaving the most destructive mastectomies to the bigger late-diagnosed ones. It should be noted that since the prognosis is directly related to the disease stage, women who will benefit from less mutilating interventions will also have a greater probability of survival, while others will not only undergo heavier interventions but will have a worse prognosis too. Guidelines for surgical breast cancer treatment include a series of interventions in strictly framed protocols. The most common techniques are the following: 1) breast biopsy and tumorectomy: the biopsy consists in the removing of tissue whips. It may be incisional if it is limited to the removing of a small part of a larger tumor, or excisional when it removes en bloc all the tumor. Biopsy is always associated with the histological examination that may be extemporaneous if the pathologist prepares the anatomical part at the freezer microtome examining it as soon as it is sent to him by the surgeon, or deferred when he looks at it later. Tumorectomy is the removal of the breast tissue with the tumor, with or without the skin covering it. It is a really therapeutic intervention that is indicated for benign tumor forms or for very limited malignant tumor forms. It is distinguished from excisional biopsy because while this is an intervention aimed at diagnosis, tumorectomy is a therapeutic intervention; 2) enlarged tumorectomy: it corresponds to the excision of the part of the breast gland containing the tumor together with at least 1 cm of healthy surrounding parenchyma. This operation is practiced for very small tumors. When they are not palpable, the surgeon to locate them follows the path of a metallic tile previously introduced under ultrasound or stereotactic guidance. In order to be sure to have made the complete exeresis of the diseased area, the anatomical sample is subjected to a radiographic control that allows the immediate comparison of the removed area (tissue around the metallic tile) with the one recognizable in the radiogram made before the intervention; 3) quadrantectomy: this operation has been proposed by Umberto Veronesi of the European Institute of Oncology (IEO) in Milan for the initial stages. Quadrantectomy refers to the removal of a piece of breast gland with the above skin and the underlying large pectoral muscle fascia. In small breasts, the exeresis can coincide with one of the four quads in which anatomically we can divide the breast. In the bigger ones, it corresponds to the removal of a breast clot. It is a limited intervention that gives excellent aesthetic results Preface xi comparable to those of a reductive mastoplasty that often require the remodeling of the contralateral breast which has become asymmetrical as compared to the operated one; and 4) mastectomy: there are some variants of this operation. Radical mastectomy is the complete removal of the breast along with the large and small pectoral muscles and the exeresis of the axillary lymphatic chain up to the third level. Surgery is the main but not the only therapeutic moment. Additional adjuvant therapies, such as hormonal, chemotherapy, radiotherapy, which help the body to fight the likely micrometastases already present at surgery, may be associated to it based on a series of further evaluations. The indications and the type of adjuvant therapy are evaluated based on the state of involvement of lymph nodes (invasion or not), on the study of hormone receptors, and on age and menopausal status of the woman. A separate case is represented by tumors in a particularly advanced phase, corresponding to Stage IV, which were very common in the past but still occur today. In these cases, it is advisable to submit the patient to some chemotherapy cycles or to radiotherapy aimed at reducing the tumor mass and hence only later, if in case, to radical mastectomy. Therapy made before surgery is called “neoadjuvant therapy.” Plastic surgery has the purpose of remedying some breast defects such as: 1) hypertrophy: it is the enlarging of one or both breasts. It is corrected with reductive mastoplasty interventions, i.e., the removal of part of the gland and of the excess skin in order to obtain the desired volume, followed by the repositioning of the nipple-areola complex (NAC); 2) ptosis: it is the lowering of the breast. Its correction consists of mastopexy techniques to restore the breast to the upper position with repositioning of the NAC; 3) hypoplasia: it is the failure of the development of one or both of the breasts. In these cases, breast augmentation interventions are based on the insertion of breast implants (containing silicone gel) with appropriate volume, shape, and profile; and 4) hypotrophy: it is the loss of tone and volume of one or both breasts due to various causes: pregnancy, lactation, elderly age, weight loss, etc. It can be corrected with mastopexy interventions associated with breast augmentation. This kind of surgery, which in most cases responds to requests of a purely aesthetic nature, is therefore also known as “aesthetic surgery.” Reconstructive surgery is instead used to reconstruct the breast when it has been necessary to remove it for neoplastic pathologies or when it has been destroyed by traumatic events. In general, the first phase is the reconstruction of the mammary mass to which, after some time, follows the reconstruction of the NAC. The choice of the surgical technique depends on several factors, including, among the most important ones: age, degree of motivation of the patient, and, in case of removal for cancer, the extent of the mutilation and the current stage of the disease. Thus, it is worth to emphasize the importance of an ethically and psychologically correct approach to the oncological patient. The therapist’s planning, which could also include total mastectomy, and which must be thoroughly explained to the woman, will have the urge to insert the reconstructive moment as the possible, if not xii Ciro Comparetto and Franco Borruto natural, conclusion of the surgical path. In this way, he/she will get a more convinced informed consent from the patient but, above all, will help her to overcome the psychological, even before physical, trauma of the mutilation she is going to undergo. The mode and time of the reconstruction are essentially related to the type and extent of the loss: 1) quadrantectomy: it is a sort of reductive mastoplasty that requires only a few simple technical measures during the wound closure. Rather, the volumetric reduction of the operated breast creates a positional and shape asymmetry with respect to the contralateral breast that is often remodeled in later times; 2) subcutaneous mastectomy: it involves removing of the mammary gland by maintaining the muscular and skin planes. The ideal surgery is a breast reconstruction that is carried out with the immediate placement of a prosthesis; 3) Halsted’s radical mastectomy: it involves a severe mutilation for the loss, together with the breast, of the two pectoral muscles. This makes it impossible to insert a prosthesis and requires a real reconstruction that is obtained by resorting to large muscle-skin edges, portions of the abdominal and large dorsal muscles covered by the skin and provided with vascular peduncles that are moved from their original site; and 4) radical mastectomy modified by Patey or Madden: these mastectomies, compared to the previous one, retain partially or completely the muscular plane. Madden’s technique, in particular, requires the immediate placing, below the large pectoral muscle, of a skin expander that will progressively be inflated, helping to create a space where, once the expander is removed, a definitive prosthesis can be inserted. Once the breast is reconstructed, after some times, the NAC is reconstructed too. For this purpose, it is possible to use some of the analogous contralateral structures or tissue areas taken in other places of the body (inguinal bend, thigh root, large lips, etc.). Keywords: breast cancer, breast surgery, plastic surgery, aesthetic surgery Introduction The obstetrician/gynecologist is the primary physician to women. The breast is an organ of reproduction, and complete breast examination is part of the obstetric and gynecological examination. In addition to history and physical examination, the obstetrician/gynecologist should be prepared to undertake simple diagnostic studies. Cancer, on the other hand, presents many challenges. The appropriate role for the obstetrician/gynecologist is one of surveillance and as a resource for patients, including the discussion of risk factors and treatment alternatives [1]. The American Board of Obstetrics and Gynecology (ABOG) has recognized the special role of the obstetrician/gynecologist in the diagnosis and treatment of breast disease and indicated in 1985 that it would require a knowledge of breast disease in its certification process. It was also recommended that the obstetrician/gynecologist provide adequate information concerning screening and perform simple diagnostic studies including aspiration of cysts, fine-needle aspiration (FNA), and appropriate follow-up studies for patients treated for breast cancer. Because open biopsy often becomes part of the treatment for breast cancer, the ABOG stopped short of recommending that every obstetrician/gynecologist perform this procedure. The American College of Obstetricians and Gynecologists (ACOG) also has increased its efforts to more clearly define the role of the obstetrician/gynecologist in the diagnosis and treatment of breast disease. Questions have been raised concerning the lack of national standards to evaluate training in the diagnosis of breast disease surgery, and the ACOG has convened many workshops to address these issues [2]. The designation of the obstetrician/gynecologist as a principal physician for women may impose new responsibilities on them, but it merely reaffirms what most obstetrician/gynecologists have long regarded as standard practice. The gynecologist has always insisted that breast examination is an integral part of the evaluation of every patient and the basic knowledge of breast disease is certainly as xiv Ciro Comparetto and Franco Borruto essential as any understanding of obstetric and gynecological problems. The obstetrician/gynecologist should follow certain guidelines that will help achieve in making an early diagnosis. These include: 1) integrate breast examination into the routine gynecological examination of all patients; 2) instruct patients in the technique of life-long, periodic breast self-examination; 3) develop proper ambulatory surgical facilities suitable for performing breast biopsies; 4) perform biopsy for all true, solid, and three-dimensional (3-D) masses: the final diagnosis of a pathological condition rests on a careful histological examination of a biopsy specimen; 5) encourage research, both basic and clinical, and etiology, diagnosis, and treatment of breast lesions, including innovative screening programs for highrisk patients; and 6) include obstetric and gynecological residency programs with specific instructions in early breast cancer detection techniques. Previously, breast cancer was viewed as a stereotype disease that progressed from the breast to the nodes to the systemic area. This has changed: breast cancer is now viewed as a systemic disease, which spreads to local and distant sites at the same time. Breast cancer is best viewed as occultly metastatic at the time of presentation. Therefore, dissemination of tumor cells has occurred by the time of surgery in many patients, and it is not surprising that radical mastectomy and local irradiation do not prevent metastatic disease [3]. Breast cancer is the leading cause of death in women between the ages of 35 and 54. It is estimated that the specialist in obstetrics and gynecology provides health care for more than half of the female population and should become familiar with both benign and malignant breast conditions. Confronted with the misconception that the disease appears in women “at risk” only and the estimate that one in every nine women born in the United States (USA) will develop breast cancer, adequate education, screening, examination, and documentation are essential in the field of breast disease [4]. Most gynecologists have accepted their role in reducing mortality from breast cancer giving a thorough breast examination to all patients. They must also be involved in the interpretation and treatment of symptoms, the use of diagnostic aids, the recognition and understanding of pathology, and in following and counseling those who have been treated for cancer. Many of the surgical procedures are within their technical abilities. Specialty organizations and residency training programs must assist in bringing the gynecologists’ educational level up to their degree of involvement [5]. Introduction xv An understanding of breast pathology is essential when caring for women with breast disease [6]. Breast diseases are a common aspect of primary care practice [7]. There has been significant improvement in breast health care over the past few decades, primarily due to advances in health research by diverse teams of basic scientists, physicians, pharmacists, industries, nurses, and social workers. This research has involved inquiries about fundamental biological alterations in breast cancer, differences in diagnostic modalities and treatment options, and various outcome studies. Increase in public awareness of breast cancer, an interest in women’s health issues, advances in radiological imaging, development of new chemotherapeutic agents, and the availability of molecular genetic testings have brought remarkable opportunities to a new insight in breast cancer. These efforts have resulted in earlier detection and prolonged disease-free intervals. However, the overall survival time has remained the same. This is mainly attributable to the wide range of individual therapy for breast cancer, which responds to a range of disease curable by surgery alone to one refractory to treatment and marked by rapid metastatic progression. Challenges remain in fostering adequate funding for biomedical, as well as behavioral and social research. Attempts should also be made to promote clinical and population-based studies and to emphasize the value of effective delivery of health-care services to all women with benign, high-risk, premalignant, and malignant breast disease [8]. Initial diagnostic errors are related to the presumption that symptoms or findings are due to benign causes. Physical examination augmented by mammographic study will disclose those benign-appearing lesions which may harbor a carcinoma. All palpable lesions and mammographically suspicious areas require that their identity is determined. The final resolution depends upon biopsy. Missing the tumor also causes diagnostic error. An accurate biopsy or “sample” must be obtained for study. Negative results of sampling techniques should be followed by formal biopsy. Failure to eliminate the primary disease by leaving tumor or breast tissue behind increases the incidence of recurrence. Total mastectomy reduces this risk. Removal of the axillary lymph nodes (ALN) also aids in obtaining cure, as well as providing prognostic information. Well intentioned attempts to obtain a better cosmetic appearance or to reconstruct the breast are secondary to the patient’s chief need which is to achieve cure. Complications of the surgical procedures are due to improper flap dissection, desiccation and trauma to tissues, incomplete hemostasis, and inadequate drainage. Attention to operative details and adherence to well established surgical principles will minimize complications [9]. There have been many changes in the last 40 years in breasts surgery. Careful consideration is given to minor points in operative technique and patient selection, which will help to achieve consistently good results with minimal complication. Great emphasis is placed on tailoring the operations to the needs of the patient [10]. Procedures for management of breast disease are those that are done most commonly by the general xvi Ciro Comparetto and Franco Borruto surgeon and for which the ambulatory surgical unit is apt to be the preferred site [11]. The function of the surgeon remains the same relative to the management of primary breast cancer. What has changed is our perception of the biology of the disease and our understanding of data evaluation and analysis [12]. Breast surgery has evolved as a subspecialty of general surgery and requires a working knowledge of benign and malignant diseases, surgical techniques, shared decision-making with patients, collaboration with a multidisciplinary team, and a basic foundation in surgical ethics. Rapid continuous changes in breast oncology practice have further substantiated dedicated expertise in breast surgical oncology. Training programs are structured to develop proficiency in fellows for advanced surgical techniques and clinical decisionmaking as well as exposure to the multidisciplinary aspects of breast cancer management. Components of a successful program include an intense multidisciplinary curriculum, engagement in clinical research, and attention to strong mentorship. National curriculum and training requirements as well as supplemental resources assist in standardizing the fellowship experience. As surgical training and the field of breast oncology continues to evolve, so do fellowship training programs to ensure high quality breast surgical oncologists equipped to deliver high quality evidence-based patient care while continuing to drive future research and trainee education [13]. Ethics is defined as the practice of analyzing, evaluating, and promoting best conduct based upon available standards. As new information is obtained, or as cultural values change, best conduct may be redefined. In 2014, the Ethics Committee of the American Society of Breast Surgeons (ASBrS) acknowledged numerous ethical issues, specific to the practice of breast surgery. This independent review of ethical concerns was created by the Ethics Committee to provide a resource for ASBrS members as well as other surgeons who perform breast surgery [14]. Breast cancer management requires a multidisciplinary approach that is tailored to the patient’s stage at presentation, desire for breast conservation or reconstruction, estimation of risk of recurrence, and assessment of the benefits and toxicities of potential adjuvant therapies. Breast surgeons, plastic surgeons, radiation oncologists, and medical oncologists staff the breast cancer treatment clinic (breast unit or breast center), and work closely together to formulate treatment plans that will optimize the likelihood for cure with an acceptable cosmetic result. This involves careful preoperative work-up, surgical axillary staging, breast irradiation in the setting of breast conservation, and selection of chemotherapy or hormonal therapy if appropriate. Newer aspects of breast cancer care, including sentinel lymph node biopsy (SLNB), post-mastectomy radiation therapy, expanded use of hormonal therapy in younger women, new agents and chemotherapy combinations, and autogenous reconstruction techniques, have become an essential part of the multidisciplinary clinical approach [15, 16]. The enormous burden placed by this disease both on the population and on health care systems explains the Introduction xvii increasing efforts and resources that have been devoted over the years to the search for a systematic and optimized strategy in breast cancer diagnosis and treatment. Today, the breast unit model is identified as the gold-standard to ensure optimized patient-centered and research-based clinical services for breast cancer patients improving survival rates and patients’ quality of life by a multidisciplinary approach in breast care [17]. The European Society of Mastology (EUSOMA) Working Group published in 2000 guidelines for the development and operation of state-of-the-art multidisciplinary breast units. The requirement of a breast unit was a quality assurance plan with the overall goal of improving breast care for women with both benign and malignant disease. Moreover, the European Parliament issued a report on breast cancer in 2003 demanding the establishment of European guidelines for the certification of breast units to prevent an inflation of breast units without the required quality standards [18]. Breast centers are defined as focused multidisciplinary facilities of excellence, dealing with the complete range of breast problems [19]. Comprehensive breast centers directed by full-time breast imagers have a significant impact on medical care from the perspective of the patient, her physicians, and the health care system [20]. The breast care continuum is characterized by a wide variety of procedures and pathways leading to similar outcomes. This variation leads to very substantial differences in costs and patient experience, dependant upon physician choice of procedure or pathway. The breast center is uniquely positioned to develop disease management for the breast, including subspecialized care, the adoption of evidence-based protocols, and comprehensive management of outcome information. This approach to disease management focuses on physician excellence and is the key to increasing the quality of patient care, decreasing the overall cost of breast cancer, and increasing reimbursement and practice satisfaction for breast physicians [21]. Over the last 40 years of breast center development, it has been found that for every obstacle there is an option for resolution. The solutions are not uniform in each setting, but rather they reflect the medical staff politics, interests, and strengths, the resources and commitment of the institution, and the needs of the community. The most important message is to validate local medical staff issues as legitimate concerns. The crucial obstacle is the lack of cooperation among the medical specialties and the lack of trust between the medical staff and management. The obstacles need to be appropriately addressed by the creation of an effective organizational structure that is successful in the local environment and by the programmatic development that reflects the medical staff’s interests and strengths. This will ensure comprehensive breast center success in the local political environment and ultimately translates into enhanced expertise, pride, and higher quality patient care [22]. The pathologist’s contribution to the interdisciplinary team approach in breast cancer management is a cornerstone for decision-making. Continuous communication with the radiologist, surgeon, oncologist, and radiation oncologist is essential. Preoperative diagnosis, surgical specimen work-up with size determination, xviii Ciro Comparetto and Franco Borruto SLNB, processing forms to facilitate the work-up, and diagnostic templates are some of the items of the pathologist’s contribution to the interdisciplinary team in breast cancer. Use of diagnostic templates or synoptic reports routinely provide the complete diagnostic information required for treatment decisions [23]. However, surgeons retain the central role in the multidisciplinary breast cancer patient care. In fact, the role of the surgeon remains important in the diagnosis, staging, and treatment of disease, as well as in educating the patient as to her best options. While technical details of the operations for these patients remain important, effective evidence-based decision-making may be even more so. Advances in the methods of breast cancer diagnosis, localization techniques, and surgical therapies, as well as the expanded role of the surgeon in breast cancer prevention, radiation therapy, and the treatment of distant disease, requires surgeons to stay up to date with the available evidence [24, 25]. Several modalities and areas of expertise are critical for optimal patient management. The basis for medical decisions and recommendations must reflect outcomes and clinical trial data that are designed and interpreted with broad input across different fields. Hence, there has been a trend for specialization in breast disease in many large community and academic practices. Furthermore, a system of communication and standardization of data values, procedures, and protocols has begun, but needs much further development. There are many natural barriers to the process of multidisciplinary care and research in terms of logistics, finances, and education. The example of preoperative neoadjuvant therapy for earlystage locally advanced breast cancer is one that involves multiple disciplines in the formulation of a treatment and in future research that will define the optimal individualized approach. This process can also shed further light on biological principles and potential for improved treatment. Solutions for overcoming barriers to multidisciplinary care should include incentives for collaborative and coordinated clinical care across disciplines. A model of increased efficiency because of pooled resources and specialization in several fields should also be accompanied by a demonstration of increased quality of care and patient satisfaction. Any process that adds to cost or inconvenience needs to be justified in an evidence-based manner. Finally, these initiatives need to be effectively communicated to the professional and policy-making communities and to the public at large through well-conceived and unbiased educational venues [26]. Chapter I Historical Overview Throughout documented history, man has admired physical beauty. Artistic works such as the Venus de Milo and Michelangelo’s David and contemporary movie stars and athletes emphasize the physical being. More than mere appreciation of corporeal attributes, ours is a reverence that perceptually bestows a halo effect of associated virtues. If certain physical criteria are met, the individual simultaneously is presumed intelligent, strong, charming, good, or any of many other socially esteemed characteristics. In a highly competitive society, it is no wonder we strive for the edge which beauty bestows [27]. Throughout the Christian era, saints have been adopted as patrons of countries and towns, trades and professions, and for protection against disease. Although patronage is usually related to the saint’s most spectacular cure or mode of death, sometimes it derives from a play on words, an unusual physiognomy, or mistaken identity. Historical research has identified 295 patrons of specific diseases. Thirteen of these saints are patrons of breast disease, St Agatha, who was martyred by mastectomy, being the most famous [28]. An ex-voto (from the Latin for “from the vow”) is an image made to express the patron’s gratitude for divine assistance in the face of personal difficulty. A late 18th century Mexican painting shows Doña Josefa Peres Maldonado undergoing a mastectomy, and, as an ex-voto, expresses her thanks for divine aid in having survived the operation. As such, the painting manifests Doña Josefa’s response to her disease, drawing on both medical and religious sources of support [29]. A Renaissance painting depicts a young woman with locally advanced breast cancer reaching out towards a healing potion in the church of Santa Maria della Grazia in Milan, that houses Leonardo da Vinci’s “Last supper.” Today’s potion may well contain neoadjuvant systemic therapy including trastuzumab that may allow breast-conserving surgery and targeted intraoperative radiotherapy [30]. 2 Ciro Comparetto and Franco Borruto Cancer was known as a disease since prehistoric times. Breast cancer is known from ancient time, and the treatment strategy evolved as our understanding of the disease changed with time. Breast cancer, seen by Galen as the commonest cancer of his time, was probably first mentioned by Hippocrates in the 5th century before Christ (BC). In 460 BC, Hippocrates described breast cancer as a humoral disease and presently, after a lot of studies, breast cancer is considered as a local disease with systemic roots. Hippocrates proposed that breast cancer, among other neoplasms, was a “systemic disease” caused by an excess of black bile. The humoral theory was further supported by Galen and dominated for centuries in medicine. A case history was described but no specific treatment mentioned. For centuries, no further cases were described, until Cato, 2nd century BC, advocated cabbage poultices for all tumors and breast cancer. Aëtius of Amida probably first described Paget’s cancer of the nipple. By the 2 nd century anno Domini (AD), treatment comprised a variety of local applications, systemic medicaments, venesection, and surgery. Surgical resection, first described by Celsus and subsequently by Leonidas (usually combined with cautery), proved curative when applied early in the disease [31]. Management of breast cancer evolved slowly through centuries in the ancient world up to the Renaissance. This period is marked by the absence of any scientifically verifiable understanding of the true nature of cancer and its natural history and consequently by a lack of effective treatment. Breast has been considered as a symbol of femininity, fertility, and beauty. Fulguration and breast amputation by using various instruments to achieve a rapid operation were widely used up to the 18th century. The Renaissance was a revolutionary period, since it stimulated medical practice: at that time, physicians started to scientifically study medicine. Vesalius greatly contributed in the advancement of surgery, and he vigorously opposed Galen’s doctrines. Many great surgeons of that time (including Paré, Cabrol, Servetto, Scultetus, Tulp, Fabry von Hilded, etc.) advanced the science of surgery. Interestingly, Bartoleny Gabrol (1590) in Montpellier advocated radical mastectomy, which was popularized by William S. Halsted 300 years later. However, the lack of anesthesia and the problem of wound infections (due to the lack of the aseptic techniques) generated significance and often problems for the surgeons of that time. Surgery was often “heroic” but primitive and even inhumane by current standards. Therapeutic nihilism was the prevailing attitude regarding breast cancer, at least among the clear majority of surgeons [32]. Today, we take for granted the blessing of anesthesia and it is almost impossible for us to imagine the agonies that surgical patients underwent in the past. The description of a mastectomy, performed in 1720 by Lorenz Heister, Professor of Surgery and Anatomy in Altdorf in the republic of Nurnberg (now part of Germany), gives a vivid idea of major surgery in those days. In the abstract from his lengthy report, which appears in the 1775 English edition of his textbook entitled “Medical, Chirurgical and Anatomical cases and Observations” he discusses the preoperative preparation, the mastectomy itself, Historical Overview 3 performed as quickly as possible and the tedious postoperative dressings of the inevitably suppurating wound [33]. The lesion was probably a massive cystosarcoma phyllodes rather than infiltrating carcinoma of the duct. His thorough description reveals that patients and surgeons have changed little in three centuries [34]. Professor Bernard Peyrilhe occupied the chairs of surgical chemistry and medical materia at the Faculty of Medicine of Paris. He was a great surgeon, cancer specialist, and historian of medicine. He led in-depth studies on cancer and realized the first experimental transmission of cancer by injecting extracts of breast cancer into an animal [35]. During the 18th and 19th centuries, management of breast cancer was greatly improved. The humoral theory of Galen, which dominated for centuries, was fallen into disfavor. ALN involvement was recognized as an adverse prognostic factor, while LeDran, in the middle of the 18th century, proposed the theory of lymphatic spread of breast cancer. He also favored the idea that breast cancer at its earliest stage was a local disease, which could be effectively treated by surgery. The need to excise enlarged ALN was recognized by other surgeons of the 18th century, including Petit, who proposed a procedure very similar to radical mastectomy. During the 19th century, significant advances were noted, including the development of anesthesia and antisepsis, a better understanding of the biology of cancer, and the introduction of microscopic examination. Radical mastectomy was widely used in clinical practice by Halsted. However, this radical procedure was used by other surgeons of that time, including Meyer. Halsted was able to report very low local recurrence rates (approximately 6%), a very important achievement given the advanced stages of breast cancer when diagnosed in women at that time [36]. In 1804, Seishu Hanaoka performed the first successful surgical treatment of breast cancer under general anesthesia in the world. It preceded by 38 years C.W. Long’s trial of ether anesthesia in 1842. Hanaoka had made many efforts to develop the optimal prescription of the anesthetic “Tsusensan (or Mafutsu-To)” for almost 20 years. Finally, he succeeded in using it clinically for breast cancer surgery, on October 13 th, 1804. Hanaoka performed operations for breast cancer in a total of 156 cases, and for many other kinds of surgical procedures. He also eagerly contrived and modified many surgical instruments. Despite such a busy daily schedule, he eagerly trained and educated many students, using his own philosophy for medical management [37]. Cooper in 1840 described mammary branches from the 2nd-6th intercostal nerves, and noticed that the nipple was supplied by branches which lay close to the surface of the gland. Eckhard (1850) divided the mammary branches into superficial branches to the skin and nipple, and deep branches to the glandular tissue and nipple, but many later authors ignored those findings. After the Second World War, cosmetic breast surgery made further research critical, as surgeons strove to design operations which would retain its shape and preserve postoperative sensation. Craig and Sykes (1970) described mainly anterior branches from the 3rd, 4th, and 5th intercostal nerves passing through the 4 Ciro Comparetto and Franco Borruto breast glandular tissue and along the line of the ducts to the nipple. Farina et al. (1980) concluded that the nipple was supplied solely by superficial lateral branches of the 4 th nerve. Using improvements in dissecting technique learned from microsurgery, Sarhadi et al. (1996) found that the nipple was innervated by the lateral cutaneous branch of the 4th intercostal nerve, by two branches, one passing superficial to the gland, and the other through the retromammary space, and by variable lateral and medial additional branches from the 2nd-5th nerves. These branches came to lie superficially and formed a subdermal plexus under the areola. This account is uncannily close to Cooper’s original description: it is a reassuring, if sobering, conclusion that his early account remains one of the most reliable [38]. The understanding of the association of mammalian ovarian function with lactation was common knowledge to dairymen early in the 19th century or earlier. Beatson of Glasgow noted the cellular anatomical similarity between pregestational breast and carcinoma in premenopausal women in 1895, leading to the monumental work of Huggins in 1941 [39]. Dr. Wendell G. Scott hailed mammography as the first significant contribution to the control of breast cancer since Halsted described the radical mastectomy in 1903. Mammography has engendered innovative approaches and has supplied new concepts of breast cancer. Through the tenacity of a few physicians convinced something could be done for the breast cancer patient, the team against this dread disease has gradually, but solidly, been built and continues to grow [40]. In the early 20th century, surgeons and pathologists arrived at the conclusion that specific anatomical and cytological changes in the breast are related to a heightened risk of developing a malignancy in the future. This conclusion was directly related to a shift from macroscopic to microscopic diagnosis of malignancies, and to the integration of the frozen section into routine surgery for breast cancer. In the interwar era, conditions such as “chronic mastitis” and “cystic breast disease” were defined as precancerous, and women diagnosed with these conditions were advised to undergo mastectomy. In the post-Second World War era, these entities were replaced by “carcinoma in situ” (CIS). The recent development of tests for hereditary predisposition to breast cancer is a continuation of attempts to detect an “embodied risk” of cancer and to eliminate this risk by cutting it out [41]. The first modern case-control study was Janet Lane-Claypon’s study of breast cancer in 1926, but the design was used only sporadically in medicine and the social sciences until 1950, when four published case-control studies linked smoking and lung cancer. These 1950 studies synthesized the essential elements of the case-control comparison, produced a conceptual shift within epidemiology, and laid the foundation for the rapid development of the case-control design in the subsequent half century [42]. The history of carcinoma was for many centuries mainly the history of breast cancer. Only when in the second half of the 19th century anesthesia and antisepsis had enabled Historical Overview 5 surgery to treat certain internal carcinomas as well, interest in malignancies other than those of the breast sprang into being [43]. The history of breast cancer treatment is divided into periods in which different methods of treatment were dominant [44]. Surgical treatment of breast cancer has been marked by a constant evolution since the Halsted’s radical mastectomy described in the late 19th century. Then, Madden’s radical mastectomy, a breast surgery that involves the ablation of tissue with the axillary lymphatic chain preserving both pectoral muscles, has become the current standard [45]. First, surgery was too radical, then radiation was overused, soon perhaps adjunctive chemotherapy will be overused. We should continue to search for new methods such as combinations of heat and radiation that might maintain or increase the immunological resistance of the host [46]. Halsted is the 19th century surgeon whose name is most frequently associated with the radical mastectomy procedure. Halsted’s radical mastectomy was the “established and standardized operation for breast cancer in all stages, early or late.” However, this type of surgery has been performed since the 16th century. The development of radical mastectomy was a long process, and many surgeons over time have contributed valuable insights and alterations to this fundamental treatment for breast cancer. This procedure may be most commonly associated with Halsted because he promoted a meticulous operative technique, synthesized the best points in the techniques suggested by the most famous surgeons of the 19th century, and provided a scientific basis for the performance of radical mastectomy, which became the standard surgical treatment of breast cancer for nearly 100 years [47]. Later in this period, theories suggesting that breast cancer was a systemic disease at inception were championed by Bernard Fisher. This alternative hypothesis of biological predeterminism was based upon results of randomized clinical trials (RCT) comparing breast-conserving therapy with mastectomy, which showed similar overall survival outcomes. Nonetheless, data from meta-analyses suggest that inadequate local therapy can increase risk of local recurrence, which can subsequently increase mortality. Local therapy of breast cancer has evolved in the face of improved adjuvant therapies and better understanding of disease biology [48]. Misconceptions and illusions prevail in the management of breast cancer. Historical review reminds us that medical practice is commonly rooted in tradition rather than proof. The Halsted mastectomy inadvertently served the burgeoning profession of surgery in the early 20th century more than it has benefited women with breast cancer, yet more than 100 years later the operation continues to thrive. Despite evidence that mastectomy, radiation following lumpectomy, ALN dissection (ALND), or intensive follow-up surveillance have little impact on survival, these practices are adhered to tenaciously. The extent to which current treatment for breast cancer succeeds in prolonging life remains open to question. Many accepted ideas and interventions are perilously disconnected from their true merit. The imperative for doctors to do something sometimes contradicts their pledge to do no harm [49]. The operation described by 6 Ciro Comparetto and Franco Borruto Halsted in 1894 and called “radical mastectomy” represents a milestone in the treatment of breast cancer. It consisted of removal of the breast, muscles, and ALN. The preHalsted era saw attitudes ranging from the willful abstention to brutal treatments by cauterization or amputation. The introduction of anesthesia and asepsis enabled more advanced surgical attempts. The stratification of patients into operable and non-operable categories has improved surgical outcome. After attempts to extend Halsted procedure (by extended or super-radical mastectomies) proved to be of little benefit, a minimallyinvasive trend emerged gradually. It started with modified radical mastectomy by Madden or Patey that spares the muscles and was then followed by breast conservative surgery that leaves breast tissue behind. Then, SLN mapping was introduced with the hope of reducing the extent of ALND. Finally, skin-sparing mastectomy appeared to conserve skin and facilitate breast reconstruction [50, 51]. The 20th century is marked by significant advances regarding the management of breast cancer. A clear trend towards less aggressive surgical operation was constantly noted. Modified radical mastectomy gradually replaced radical mastectomy during the second half of the 20th century, while during the last two decades breast-conservation therapy became the treatment of choice for the treatment of breast cancer. This type of therapy includes segmental mastectomy (either quadrantectomy or lumpectomy) with ALND, followed by postoperative irradiation. Other significant advances during the 20th century include the introduction of systemic therapy (chemotherapy and hormonal therapy) and radiation therapy. Better patient follow-up, statistical analysis, development of staging systems and the introduction of frozen section, the development and wide use of mammography (including screening mammography), breast reconstruction following mastectomy, and the development of newer diagnostic methods [including breast magnetic resonance imaging (MRI) and the advanced breast biopsy instrumentation (ABBI)] are other advances that contributed to a better management of breast cancer patients. SLNB has been introduced during the 1990s to reduce morbidity due to ALND. Despite these advances, breast cancer remains a significant problem and represents a field of active and intense research [52]. On January 7th, 1948, a meeting was held at the Royal Society of Medicine (RSM) in London. Its purpose was to settle a controversy. Robert McWhirter, an Edinburgh-based radiotherapist, had been invited to defend the scandalous position advocated by Geoffrey Keynes ten years previously: that radical mastectomy offered no survival advantage when compared to simple mastectomy plus local radiotherapy. The negative publicity surrounding the meeting proved overwhelming for Keynes and he abandoned his research. Indeed, the events of the meeting may have been quietly buried were it not for McWhirter who, over the following decade, pursued Keynes’ research. He refined his technique, sparing patients the disfiguring and painful radical mastectomy without compromising overall survival. Later, he garnered support from other researchers, which led to a series of papers confirming his original findings. Historical Overview 7 Towards the end of his career, he also made contributions to service organization and hormone therapy, eventually holding the Presidency of the Faculty of Radiologists. By keeping the controversy alive, McWhirter was instrumental in overturning 60 years of surgical dogma. He remains a pivotal figure in the history of breast cancer [53]. As we have said, since the 1950s the treatment of breast cancer has changed substantially. This related surgery has become less disfiguring without either impairing survival or increasing recurrences. Adjuvant chemotherapy has also contributed [54, 55]. Considerable controversy still exists regarding the correct management of breast cancer [56]. In fact, during the past 100 years there have been two major controversies about the treatment of primary breast cancer. The first controversy, which occurred approximately 40 years ago, questioned the performance of radical mastectomy, as originally proposed by Halsted in the 1890s. That controversy was resolved using laboratory and clinical research, hypothesis formulation, and evaluation of the efficacy of the latter through the conduct of RCT. A second major controversy arose when MRI began to detect the presence of tumor multicentricity in many breast cancer patients, resulting in a resurgence in mastectomy in women who could have been treated with breast-preserving surgery. Because the use of science resolved the first controversy, there was scientific evidence to justify the current reversion to mastectomy. Extensive examination of the vast amount of recent medical literature related to that subject, that is, individual articles, review articles, and reports from the use of RCT, demonstrated that many physicians are not familiar with the scientific method, and thus, were unable to present, in those articles, credible evidence to support mastectomy in the presence of tumor cell multicentricity. Aside from the RCT conducted by the National Surgical Adjuvant Breast and Bowel Project (NSABP) begun in 1976, which demonstrated no statistically significant difference in disease-free survival, distant disease-free survival, and overall survival between mastectomy and lumpectomy with or without radiation therapy, there has been no information in any of the few recently conducted studies involving multicentricity to justify the current resurgence in mastectomy [57]. Until the 1960s, the central public health message about breast cancer was that women should not delay seeking medical attention for breast problems. Problematic assumptions about the natural history of cancer, the efficacy of surgery, and individual responsibility for disease contributed to the durability of the “do not delay” message. More important, the message catalyzed or sustained changes in the routines of ordinary women, general practitioners, surgeons, and pathologists, which led to the perception that the campaign against cancer was working. Thus, a powerful set of reinforcing perceptions and behaviors maintained the centrality of the “do not delay” campaign until the era of mammography [58]. Most of the period from 1900 to 1970 was governed by the “non-science” of anecdotalism and classical inductivism and was marked by the absence of a scientific gestalt. In keeping with the Halstedian concept that breast cancer was a local disease that 8 Ciro Comparetto and Franco Borruto spread throughout the body by contiguous extension and could be cured by more expansive surgery, the disease was treated with radical surgery. In 1950, however, a new era of enlightenment began to emerge. The awareness that there was a scientific process in which hypotheses generated from laboratory and clinical investigation could be tested by means of RCT was a seminal advance, as were findings from studies that laid the groundwork for the modern era of steroid hormone action, including identification of estrogen receptors (ER). Expanding knowledge regarding tumor cell kinetics, tumor heterogeneity, and technological advances related to mammography and radiation therapy were also to play a role in making possible the advances in therapy that were subsequently to occur. In the past 30 years, as a result of laboratory and clinical investigation, the Halstedian thesis of cancer surgery was displaced by an alternative hypothesis that was supported by findings from subsequent clinical trials. A new paradigm governed surgery for breast cancer, and lumpectomy followed by radiation therapy became accepted practice. A second paradigm that governed the use of adjuvant systemic therapy arose because of laboratory and clinical investigation. Treating patients who were free of identifiable metastatic disease with systemic adjuvant therapy because some of them might develop distant disease in the future was a revolutionary departure from prior treatment strategy and became a new exemplar. Not only did chemotherapy favorably alter the outcome of breast cancer patients, but the antiestrogen tamoxifen (TMX) benefited patients with all stages of the disease. TMX also reduced the incidence of contralateral breast cancer, as well as tumor in the ipsilateral breast following lumpectomy. The use of preoperative therapy was also found to enhance breastconserving surgery in women with large tumors, although its value in other circumstances is still being defined. The observation that, because of TMX administration, invasive and non-invasive breast cancers can be prevented in women who are at increased risk for such tumors, and the finding that pathological entities such as atypical hyperplasia, lobular CIS (LCIS), and intraductal CIS (DCIS) can identify women who should be considered candidates for TMX serve as a fitting capstone to the accomplishments of the 20th century. Breast cancer prevention has now become a reality. Unfortunately, a variety of circumstances have arisen as the result of advances in the understanding and treatment of breast cancer over the last 50 years that threaten to nullify the progress that has been achieved. This distressing phenomenon may be reviewed as a “paradox of accomplishment.” The numerous uncertainties, issues, and questions that have arisen following the report of each advance in treatment, the surfeit of new information that has not yet been integrated into treatment strategies, the undesirable consequences of enhanced tumor detection, a reversion to Halstedianism and anecdotalism, and the uncertainty of therapeutic decision making resulting from the demonstration of small but statistically significant benefits, particularly in patients with good prognosis, need to be addressed. Inappropriate interpretation of those Historical Overview 9 circumstances threatens to deny women with breast cancer and those at high risk for the disease the opportunity to benefit from treatments that have been proven to be of worth [59]. Umberto Veronesi’s contribution was to offer the same possibility while considerably reducing the mutilation that went with cure. Large-scale clinical trials conducted by Veronesi’s group in Milan in the 1970s and 1980s to demonstrate the efficacy of quadrantectomy, radiotherapy, and ALND (QUART) as conservative treatment for small-size breast cancer set in motion the world-wide trend to conservative surgery in all forms of cancer [60]. Breast conservation surgery has become established but is always flanked by additional local treatment, and often by systemic therapy. Studies conducted in Milan were the first to demonstrate that conservative surgery plus an adjuvant therapy such as radiotherapy is efficacious in treating small-size breast cancer. As the year 2000 is passed, it has become clear that breast cancer is curable in a high proportion of cases and attention is turning to improving the aesthetic outcome of surgery, and to investigating the biological and genetic factors that influence the disease [61]. Today, the patient with newly diagnosed breast cancer and her surgeon have significantly more varied treatment options. Radical surgical resection has been supplanted by breast conservation therapy. Biopsy methods and the actual surgical techniques continue to be refined. Further developments have emerged in the debates over the efficacy of ALND and SLNB. Diverse differences are seen in breast cancer of younger patients due to some fundamental distinctions in their disease. As we entered the new millennium, breast cancer is curable in a large percentage of women. While attention is turning to the investigation of the biological and genetic factors involved with this disease, surgical regimens maintain a pre-eminent role in the overall quest for cure [62]. As we have seen, the current management of breast carcinoma is very different than it was a relatively few years ago. The improvements in care have been introduced into practice because of RCT. From the non-randomized but controlled clinical trial of radical mastectomy by Halsted demonstrating that loco-regional control could be dramatically improved, trials have become considerably more sophisticated in both design and analysis. RCT of breast conservation therapy were begun in Milan by Dr. Veronesi. Trials by the NSABP headed by Dr. Bernard Fisher have, with other trials, defined the benefits and techniques of this less morbid treatment approach. Improvements in survival have also emerged from RCT of adjuvant systemic therapy. Although these improvements are significant, they do not eliminate the risk of death from breast cancer. Much remains to be answered in the clinical management of breast cancer. RCT will assess new developments and introduce improvements to clinical practice. Funding for research in breast cancer must support basic investigation into the biology of the disease process, new approaches to control disease, and RCT to test therapeutic principles and 10 Ciro Comparetto and Franco Borruto techniques [63]. The mastectomy that is performed today is a procedure born from hundreds of years of discoveries, inventions, and amendments to existing surgical techniques. The reasons for performing this extreme surgery have changed as well, ranging from unilateral breast removal to allow greater upper limb functionality to bilateral removal of the breasts or breast tissue in individuals predisposed to breast cancer or in individuals who have already been diagnosed. The additions of surgical tools and anesthetics to the field of medicine further transformed the surgical field in general and had a large impact on the mastectomy. Halsted’s radical mastectomy served as the basis of most future breast removal techniques, and is the method recognized today as the “radical mastectomy.” Most radical surgeries are currently used for prophylaxis, whereas less invasive lumpectomies have eclipsed breast removal surgeries as of the latter half of the 20th century [64, 65]. Artificial X-rays were first produced in Germany in 1895 and used for cancer almost immediately. During the century since this remarkable discovery, radiation therapy has now become the most important non-surgical modality in cancer: over 50% of all cancer patients now receive radiotherapy at some point during the illness. Radiation therapy has increasingly replaced surgical resection for primary control of a variety of solid tumors, particularly where surgical excision is accompanied by severe long-term tissue loss or psychological morbidity. Frequent examples include breast cancers. Combinations of surgery and radiotherapy are increasingly used, for example in the preferred management of most breast cancers, by wide local surgical excision, breast preservation, and postoperative radiotherapy. In other circumstances, radiation is routinely combined with chemotherapy. In 1990, almost half a million patients were treated with radiation therapy in the USA. Recent technical advances, both in imaging and therapy beam precision, have greatly improved the therapeutic ratio and accuracy of modern radiotherapy [66]. The radiation treatment of breast cancer has evolved from 2-D to 3-D conformal and to accelerated partial breast irradiation (PBI), aiming to reduce normal tissue toxicity and overall treatment time. Systemic therapy in the form of hormone therapy, chemotherapy, and biological agents is now a well-established modality in treatment of breast cancer. The current perspective of breast cancer management is based on the rapidly evolving and increasingly integrated study on the genetic, molecular, biochemical, and cellular basis of disease [67]. Endocrine surgery includes excision of diseased or sometimes normal endocrine glands and occasionally the transplantation of endocrine tissues. Male castration was performed for social reasons in prehistoric times, and thyroid operations were described during the 12th century. Until the end of the 19th century, most operations were undertaken to relieve the local effects of pathological enlargement of the thyroid, ovaries, pituitary, and adrenals, and with the development of anesthesia, antisepsis, and effective hemostasis, thyroidectomy for benign, non-toxic goiter was perfected. After the Historical Overview 11 discovery of hormones early last century, knowledge of endocrinology increased, and many syndromes of hormonal excess were described. Surgeons began to operate to relieve them. Results improved: 1) with mastery of surgical technique, especially for operations on the thyroid, parathyroids, and pituitary; 2) with the development of methods for diagnosis of syndromes and the localization of lesions; 3) with teamwork; and 4) with the use of hormones, drugs, and radiotherapy as alternative or additional forms of therapy before, during, and after operation. For about 60 years, increasing numbers of surgeons have specialized in endocrine surgery as a discipline within general surgery, and results of treatment have improved greatly [68]. Endocrine therapies for breast cancer have been used for more than a century. The concept that changing the hormonal balance of the patient with breast cancer could lead to changes in tumor growth and regression of metastatic disease was recognized even before hormones and endocrine agents were available. Ablation of ovaries, adrenals, or hypophysis was used in advanced disease to obtain tumor regression and control of symptoms. Ovarian ablation was also tested for operable breast cancer showing a significant beneficial treatment effect. Several endocrine agents have been developed in recent years: estrogens, androgens, progestins, antiestrogens, aromatase inhibitors, gonadotropin-releasing hormone (GnRH) analogues, antiprogestins, and antiandrogens. The use of some of these agents in advanced disease led to investigations in early breast cancer: TMX was the drug which was most extensively tested, showing a significant long-term benefit for treated patients. Progestins [medroxyprogesterone acetate (MAP)] and aromatase inhibitors (aminoglutethimide) were also tested in a few clinical trials, but no conclusive recommendations for their use in patients with operable disease may be formulated. The most important current challenges for the appropriate use of endocrine therapies in breast cancer include: 1) understanding the effect of endocrine therapies and the mechanisms of resistance associated with their use; 2) developing new agents with novel endocrine antitumor effect; 3) defining the best way to combine endocrine agents with cytotoxics or with other endocrine manipulations; and 4) identifying long-term effects of endocrine agents in terms of disease control and prevention, as well as desirable and undesirable side effects [69]. 12 Ciro Comparetto and Franco Borruto One hundred years ago, ovarian ablation was shown to be an effective treatment for advanced breast cancer in premenopausal women. Since that time, many different treatment modalities have been advocated to improve patient survival. The value of adjuvant ovarian ablation, however, has recently been established in the overview of breast cancer clinical trials. In fact, comparison of the efficacy of combination chemotherapy with earlier trials of oophorectomy demonstrate the superiority of oophorectomy. The effectiveness of chemotherapy may largely be the result of partial ovarian ablation produced in premenopausal patients. The use of TMX maintenance in oophorectomized women might provide an optimal therapy for the control of breast cancer recurrence [70]. After 100 years of using hormonal therapy for the treatment of breast cancer, and developments since 1942 in chemotherapy, combining the two modalities seemed a logical next step. However, trials using TMX plus cyclophosphamide (CP), methotrexate (MTX), and 5-fluorouracil (5-FU), or dibromodulcitol (DBD) and doxorubicin with TMX showed no improvement in survival, and considerable toxicity. Nevertheless, it was learned from these trials that breast cancers exhibit cellular heterogeneity about ER status and that hormonal therapy and chemotherapy have different actions and toxicity. In addition, cell cycle specific agents are most effective against rapidly dividing cells. Further trials utilizing these concepts are warranted, although routine use of combined chemo-hormonal therapy is not yet recommended [71]. In conclusion, this historical perspective on breast cancer tells us how and why certain therapeutic eras have reached ascendancy and then declined. Therapeutic revolutions occur after a crisis develops when there is a general recognition that clinical interventions are not producing positive results predicted by the prevailing paradigm. The attitude of premodern surgeons was influenced by the very real possibility of doing more harm than good by operating upon women with breast cancer. Up until Halsted, the consensus was clearly that, unless forced by the circumstances, surgical resection should be avoided for disease much more advanced than very early-stage tumors (the cacoethesis of Celsus). Twentieth century progress in antisepsis, anesthesia, and surgery changed this point of view. The first three quarters of that century saw more and more aggressive operations performed, while the last quarter century reversed this trend, with reduction of the size of breast cancer operations based largely on the teachings of Fisher. A new crisis is upon us now in that trials of early detection have resulted in unexpected disadvantages to certain subgroups and there is previously unreported structure in early hazard of relapse, clinical data that suggests the act of surgery might accelerate the appearance of distant metastases. The explanation that agrees with these results, as well as physicians of antiquity, is that surgery can induce angiogenesis and proliferation of distant dormant micrometastases, especially in young patients with positive nodes [72]. Thus, breast cancer is a daunting disease and constitutes a continuing medical health Historical Overview 13 problem through the ages for millions of women worldwide. Physicians, from the early periods of recorded history have tried to heal breast cancer patients, with results that were fairly promising at times and disappointing at others. The science of medicine evolved through the ages under the scrutiny and critical thought of the many prominent scholars and researchers of their times who constantly added to the therapeutic armamentarium. Surgeons described new therapeutic approaches, and anatomists, through their elaborate descriptions, added useful insights on the art of healing. Although the Middle Ages hindered temporarily any progress in the field of medicine, the Renaissance became the vaulting horse for science in its broadest sense. However, it was not until the 19th century, with the discovery of anesthesia, the introduction of antisepsis, and the establishment of microscopy that giant scientific leaps in the field of breast cancer treatment occurred. The 20th century, with the development of chemotherapy and radiation and the undertaking of numerous clinical trials, offered new insights regarding breast cancer management [73]. In the history of the development of the treatment of breast cancer over a 4000-year period, there have basically been three paradigms within which we have studied and developed treatment for this disease. Clinical trials over the last 40 years, as an expression of the scientific method in action, have demonstrated the limited success of the contemporary paradigm with its therapeutic sequelae of breast conserving surgery and adjuvant systemic therapy. At the same time, a critical review of the natural history of breast cancer and the results of current treatments suggest logical inconsistencies in the model. Therefore, a novel paradigm better fits the available information by suggesting that metastases are not only a result of cellular transmission of breast cancer, but subcellular transmission using the mechanism of in vivo transfection. Although this may sound farfetched, there are a series of remarkable studies in the literature which support this conceptual revolution. This is surely a fertile field for research and the therapeutic consequences would be obvious. They might suggest that more aggressive adjuvant systemic chemotherapy based on the conventional model is unlikely to achieve any additional benefit, whereas therapy based on antiviral drugs might produce the next therapeutic advance [74]. In the 1980s, breast-conserving surgery was accepted in the surgical treatment of early breast cancer and during the last years of last century SLNB has emerged as a method to avoid ALN clearance for node-negative axillae. This will lead to a diminishing number of ALN clearance procedures. Breast cancer surgery will increasingly be performed as day-case operations, under local anesthesia. The real surgical challenges during the last decade has been immediate breast reconstruction and oncoplastic breast-conserving procedures. Therefore, breast surgery will increasingly be performed by plastic surgeons [75, 76]. Plastic surgeons have been performing operations to improve the aesthetic aspect of the breast for centuries. The Amazon philosophy has been increasing in popularity because of the evolving status of women in society. Many references point to 14 Ciro Comparetto and Franco Borruto Themiscrya on the southern coast of the Black Sea in Anatolia as the Amazon homeland. The hypotheses that have been proposed to explain the method of breast mutilation include amputation, cauterization, breast searing, and breast pinching. It is generally believed that the primary purpose was to facilitate the efficient use of a bow. Another explanation would be that breast mutilation was performed for medical reasons, including the prevention of breast pain, the development of a tender lump, or cancer. There is another school of thought on this involving religious and sociological reasons that breast mutilation was a badge of honor for warrior women and a sign that a woman had become a real warrior and a sacrifice to Artemis as a sign of service. Much indirect proof and archeological evidence point to their historical existence. The Amazons, who lived in an autonomous and original social model, changed their image and function to suit the needs of the society and the times [77]. Throughout ancient times, great controversy produced many theories of how breast cancer occurred and the best treatment. Because of beliefs that closure of mastectomy sites could conceal tumor recurrence, breast reconstruction did not gain wide acceptance until the mid-1900s. In fact, although the need for mastectomy has been evident for many years, postmastectomy reconstruction has been recognized as an achievable outcome for only a little over a century. Today, plastic surgeons have a variety of techniques to reconstruct the breast. The earliest attempts at reconstruction used autologous techniques that were either unsuccessful, not reproducible, or were associated with significant morbidity. The first autologous muscle flap for breast reconstruction was the latissimus dorsi myocutaneous flap, described in 1896 by Iginio Tansini. The introduction of Carl Hartrampf’s transverse rectus abdominis myocutaneous (TRAM) flap and Robert J. Allen’s deep inferior epigastric artery perforator (DIEP) flap have also provided excellent reconstructive options. The myocutaneous flaps that are being used for breast reconstruction have a long history, although the techniques of today are more sophisticated than those of the past. Louis Ombredanne was the first to describe the pectoralis muscle flap for immediate breast mound reconstruction in 1906. Breast reconstruction was not encouraged in the early part of this century. Lately, the advances in the myocutaneous flap have made breast reconstruction without a prosthesis possible. The tissue that was transferred to reconstruct a breast using a tubed pedicle flap in multiple stages can now be transferred in a single stage with a better result. Advances in reconstructive surgery led to a revival in autologous techniques for breast reconstruction, with microsurgical free-tissue transfer potentiating a new range of potential donor sites. The abdominal wall became the donor site of choice, and with the advent of perforator flaps, morbidity associated with flap harvest was minimized. In cases where the abdominal wall is unsuitable, flaps such as the superior and inferior gluteal artery perforator (GAP) flaps, the musculocutaneous gracilis flap, and the “stacked” DIEP flap Historical Overview 15 are frequently used options. Recent developments in endoscopic instrumentation have enabled surgeons to perform many types of procedures through small incisions located at a distance from the surgical site. The transumbilical breast augmentation (TUBA) has advantages over other methods, including a quicker recovery, less pain, and lower chances of complications. The development of minimally invasive techniques for implant placement and flap harvest, such as endoscopy, continue to evolve, and research in tissue engineering offers a vision for a future without the need for a donor site. About augmentation, Vincenz Czerny from Heidelberg attempted to enhance a woman’s breast in 1895 with implantation of a lumbar lipoma. Soon after, surgeons used paraffin injections and polyvinylic alcohol sponge implantation, which yielded disastrous results. Prosthetic techniques became sought after, with silicone prostheses widely used until concerns about potential adverse effects led to the investigation of alternate options. With these concerns shown to be unfounded, silicone and saline prostheses evolved with successive generations of implants. In 1961, Thomas Cronin and Frank Gerow promoted the first silicone implant, paving the way for today’s silicone and saline prototypes. Since then, a variety of non-silicone materials have been injected or implanted to augment or to reconstruct the hypoplastic female breast, including autologous tissues, intramammary- or submammary-injected alloplastic materials, and preformed alloplastic materials other than silicone. For various reasons, none was fully acceptable. The introduction of the medical-grade silicone bag prosthesis in the early 1960s improved the results of mammary augmentation dramatically and reduced the incidence of fibrous contracture and implant extrusion. Other methods of breast augmentation became obsolete. The introduction of the silicone gel implant in 1962 began the modern era of breast augmentation, and over the past 50 years, breast implants have evolved to encompass a wide array of commercially available implants today. In April 1992, the moratorium on silicone gel breast implants began, and much of the experience and development of the cohesive shaped implants occurred outside the USA. During this time, saline implants were used almost exclusively in North America, whereas silicone implants continued to be used throughout the rest of the world. In 2001, cohesive gel implants became available in the USA under an Investigational Device Exemption (IDE) Study. Three companies have USA Food and Drug Administration (FDA) approval of fifth-generation implants: Sientra (Sientra Inc., Santa Barbara, CA, USA), Allergan (Allergan Inc., Irvine, CA, USA), and Mentor (Mentor Worldwide LLC, Irvine, CA, USA). These implants are unique due to the texture of the shell, implant dimensions, gelshell interaction, gel fill ratio, degree of cross-linking, and form stability. Although form stability is a relative term, it may best be reserved to describe the increased ability of the implant to maintain its basic, intended shape. The cross-linking, form stability, and cohesiveness of these fifth-generation implants provide surgeons with an innovative tool to more closely create a natural breast in both shape and softness. 16 Ciro Comparetto and Franco Borruto Although reduction mammoplasty techniques had originated centuries earlier than mastopexy methods, the advancements of both have largely paralleled one another. In 1949, the Wise pattern was introduced to preoperatively plan safer and predictable outcomes in breast reductions. Efforts to minimize scars were achieved with Claude Lassus’ introduction and Madeleine Lejour’s subsequent modification of the vertical scar mammoplasty [78-82]. Erich Lexer wrote the first publication, in Spain in 1921, on the correction of pendular breasts. This author was the first in the history of mammoplasty to perform breast reduction with an “open” nipple-areola complex (NAC) transposition, with preservation of the continuity of the skin to the remaining gland. This feature was far ahead of its time, as the techniques based on this concept did not become popular until after 1955. Lexer also was the first to propose subcutaneous mastectomy for treatment of fibrocystic disease, to perform breast augmentation in the ptotic hypoplastic breast with fat flaps, and to use free fat grafts taken from the abdomen or hips for augmentation mammoplasty. In the early 1900s, the best method for enlarging small breasts was to inject them with paraffin. Within 50 years, researchers turned to the free grafting of autogenous material to achieve breast enlargement. Then came the fast and easy silicone injections of the 1950s and 1960s. For the last 20 years, the surgical implantation of alloplastic materials has been used for augmentation mammoplasty. The management of the greatest complication, capsular contracture, is difficult because it is the natural, enhanced scarring response of tissue that has been subjected to hosting a foreign body, the prosthesis. There is no simple solution to this problem, only the possibility that through manual manipulation of the augmented breast tissue, the intensity of the contracture can be minimized [83]. Chapter II Breast Biopsy Breast symptoms are appropriately evaluated by a breast-oriented history and by the diagnostic triad of clinical breast examination, FNA, and mammography. Detection and management of a breast mass requires an optimal environment for interpretation, relevant use of clinical information, technically excellent imaging procedures, proper interpretation of the findings, and pertinent recommendations. Masses suspicious for malignancy by only a single modality ideally should undergo some form of biopsy, as the consequences of delayed diagnosis of breast cancer can be devastating. Early invasive breast cancer can be identified and treated if the appropriate evaluation is performed [84]. In the case of a palpable dominant mass, concordance of the diagnostic triad yields a reliable clinical diagnosis. If there is not concordance, or if there is any doubt about the diagnosis either on the part of the physician or the patient, open surgical biopsy provides the definitive histological diagnosis. The goal in clinical practice is to detect nonpalpable cancers by ordering screening mammography for all patients eligible by age, history, or both. The long-term cancer-free survival of women treated for non-palpable breast cancer is excellent [85]. Biopsy techniques include aspiration for fluid, for cytology, or for histology. The indications for each method depend on the physical and/or mammographic findings. If a breast cancer is diagnosed, then treatment will depend on the size and location of the lesion and the patient’s wishes [86]. Concepts regarding the best methods for doing biopsies and the most appropriate methods for treatment of patients with breast cancers have changed dramatically since the early 1970s. Currently, formal biopsies (incisional or excisional) are being done as two-step procedures with reliance on permanent sections for diagnosis [87]. 18 Ciro Comparetto and Franco Borruto Patients with a palpable mass often benefit most from aspiration. As the clinician tries to aspirate, fluid may be obtained if the lesion is cystic. A solid lesion can be assessed with FNA biopsy with a very high degree of accuracy. Excisional biopsy should be used when a cystic lesion recurs, the mass does not disappear after the cyst is aspirated, or if the fluid obtained is bloody. A solid lesion may need to be excised if the aspirate is negative. The overall detection rate of breast cancer is approximately 20% for excisional biopsies. Mammographically detected lesions can be evaluated with needlelocalization biopsies and stereotactic needle biopsies (SNB). The detection rates for breast carcinoma averages approximately 30%, with at least 20% of these lesions being non-invasive. The role of core needle biopsy (CNB) of palpable lesions is limited: however, histological confirmation of positive cytological results from aspirate is possible with this approach. SNB appears to correlate well with the specimen obtained at incisional biopsy and may decrease the need for needle-localized excisional biopsy [88]. Mammography is an excellent screening tool for the detection of breast masses. One of the goals of the radiologist is to separate benign breast masses warranting no intervention from the indeterminate and malignant masses that require histological evaluation. Thorough mammographic and sonographic work-up of lesions detected at screening will reduce the number of biopsies performed and increase the true positive biopsy rate. By systematically evaluating a breast mass as to its density location, size, margins, and interval change, one can separate benign lesions from those requiring additional work-up and those that will require a biopsy diagnosis. Any features suggesting malignancy should prompt histological assessment. For many women, FNA biopsy (FNAB) or largeCNB, guided either stereotactically or sonographically, will not only reduce the morbidity associated with excisional biopsy but also decrease the cost of evaluating questionable lesions found at screening. Ultrasound examination will further reduce the number of biopsies by separating cysts from solid and indeterminate lesions that require further evaluation. The mammographer’s experience and confidence as well as patient’s compliance with follow-up recommendations will help the mammographer reach this goal [89]. In 1989, the Canadian Cancer Society (CCS) recommended that women over 50 years of age should undergo mammography as a test for breast cancer in centers dedicated to such programs. This recommendation and others by American societies have increased the number of mammographies done for screening purposes. As a result, many mammographic abnormalities are reported by radiologists, who recommend biopsy. The surgeon should review the films with the radiologist and ensure that the anomaly is real. Attention should be given to specific signs of cancer, such as spiculated lesions. However, non-specific signs of cancer, such as microcalcifications, Breast Biopsy 19 microlobulation, and architectural distortion, should be evaluated carefully before biopsy is carried out. Stereotactic FNA can decrease the number of surgical biopsies needed. The surgical biopsy should be a one-step, segmental mastectomy done for diagnosis and treatment. The specimen should be oriented, inked, and X-rayed and a definitive diagnosis made on paraffin blocks. Frozen sections should not be made of microcalcifications [90]. The development of mammography and that of breast specimen radiography have paralleled one another. The preparation of radiographs of breast specimens is obligatory when biopsy is performed for purely mammographic indications and when no palpable mass is present. Such cases are being encountered with increasing frequency because of the development of centers for breast cancer screening. Close collaboration among pathologists, radiologists, and surgeons and precise attention to procedural detail are necessities wherever this situation arises. The application of specimen radiography as a routine to all breast specimens is regarded as an adjunct to gross examination. While useful, its yield of otherwise undetected occult carcinoma is low. Whether other advantages outweigh the cost appears to be a matter for individual decision. Investigative uses of breast specimen radiography have received little attention [91]. The increasing use of screening mammography, liability risks, and volume control legislation by the federal government pose a major challenge to clinicians to safely select patients for breast biopsy. Despite a normal mammogram, a palpable breast mass often requires aspiration or excisional biopsy. Careful clinical judgment must prevail if observation is elected. A biopsy should be performed on a clinically suspicious mass whether the mammogram is suspicious or not. Thoughtful clinical judgment and interdisciplinary cooperation promote an acceptable benign to malignant ratio for breast biopsies [92]. As screening mammography has become more widespread, the urgency to provide more sensitive and more specific interpretations has also increased. Although many calcifications detected on mammograms are characteristically benign and need no further evaluation, there are many microcalcifications that warrant further evaluation with special views. The importance of their detection lies in the fact that many malignancies are mammographically manifested solely as microcalcifications. Once detected, radiologists have attempted to characterize and stratify microcalcifications by their level of suspicion to improve the predictive value of biopsy recommendations. This is an important endeavor because the cost to society, especially in this era of limited resources, and the collective anxiety produced by benign breast biopsies are both great. When a biopsy is recommended, the breast team should ensure that histopathological correlation is achieved. Communication among colleagues from different specialties should be encouraged and can be fostered by the use of the Breast Imaging Reporting and Data System (BI-RADS) lexicon (Table 1) [93]. 20 Ciro Comparetto and Franco Borruto Table 1. Breast imaging-reporting and data system (BI-RADS) classification (adapted from radiopaedia.org, 2017) BI-RADS 0: o incomplete, further imaging or information is required, e.g., compression, magnification, special mammographic views, ultrasound; o this is also used when requesting previous images not available at the time of reading. BI-RADS I: negative, symmetrical and no masses, architectural disturbances or suspicious calcifications present. BI-RADS II: benign findings, interpreter may wish to describe a benignappearing finding: o calcified fibroadenomas; o multiple secretory calcifications; o fat-containing lesions such as: oil cysts; breast lipomas; fibroadenolipoma or mixed density hamartomas; galactoceles; o simple breast cysts; o these all should have characteristic appearances, and may be labeled with confidence; the interpreter might wish to describe intra-mammary lymph nodes, implants, etc. while still concluding that there is no mammographic evidence suggesting malignancy. BI-RADS III: probably benign, short interval follow-up suggested. BI-RADS IV: suspicious abnormality: o there is a mammographic appearance which is suspicious for malignancy; o biopsy should be considered for such a lesion; o these can be further divided as: BI RADS IVa: low level of suspicion for malignancy; BI-RADS IVb: intermediate suspicion for malignancy; BI-RADS IVc: moderate suspicion for malignancy. BI-RADS V: there is a mammographic appearance which is highly suggestive of malignancy, action should be taken. BI-RADS VI: known biopsy proven malignancy. A palpable mass in a woman’s breast represents a potentially serious lesion and requires evaluation by history taking, physical examination, and mammography. The initial objective is to distinguish simple cysts from solid lesions, which can be accomplished with FNA. A solid lesion requires a firm diagnosis, and this usually calls for removing the lesion for histological examination. A positive result on cytological examination after aspiration is sufficiently accurate to justify one-stage diagnosis and Breast Biopsy 21 treatment, with confirmation by examination of a frozen section obtained during the procedure. A negative or suspicious finding on cytological evaluation is inconclusive, and outpatient biopsy is indicated. Perfection in diagnosis will require the removal of every solid mass. This can be expected to result in the biopsy of many benign lesions, but removal of many of them is desirable on other grounds. Although in some instances the probability of cancer may be exceedingly small, it is never zero. If biopsy is not recommended, the probability of cancer should be estimated so that the patient can decide whether the level of risk is acceptable to her [94]. Increased public and professional awareness has resulted in more women obtaining mammograms. As a result, the surgeon is often called on to diagnose and treat occult breast lesions. Although current controversy questions the earliest age at which screening mammography truly lowers the death rate from breast cancer, that mammography does detect breast cancer years before it might be discovered as a mass in the breast cannot be challenged. Mammographic techniques have improved so that smaller and smaller areas of suspicion may be identified, and mammographers have gained greater judgment in the interpretation of these minute radiographic abnormalities. This has inevitably led to an increase in procedures designed to explain them. The incurred costs, both emotional and economical, of positive mammographic “calls” are considerable. Whether practicing medicine as patients’ advocates or, as unfortunately currently seems to be of equal importance, exercising politically correct and cost-effective mandates, the management of non-palpable breast lesions certainly bears witness to the correlation of cognitive and procedural skills and cooperation between physicians, as well as the technical achievements of contemporary medicine [95]. The development of new diagnostic modalities has changed the way such breast lesions are approached. Management decisions are made in the context of new pressures applied by the growing managed care imperative and increased medical-legal exposure [96]. The specimen excised for the mammographically detected lesion is somewhat unique and requires special consideration by the surgical pathologist. The biopsy, in most instances, contains no grossly visible lesion and is relatively large, so that blocking in its entirety is not practical. The pathologist needs to establish that the radiological abnormality is contained in the biopsy and to this end specimen radiography is required. Established prognostic parameters for breast carcinoma, such as tumor size, histological type, grade, and hormone receptor status are equally applicable in the non-palpable breast lesion, but consideration should also be given to the identification of epithelial proliferations which have increased risk for subsequent carcinoma. In addition, the recognition of DCIS in the biopsy indicates potential for widespread disease, and LCIS, a less commonly detected lesion in mammography, is associated with multifocality and disease in the contralateral breast. Both lesions are also associated with increased risk of recurrence in the remaining breast tissue. Failure to observe the corresponding quantity of calcium seen in 22 Ciro Comparetto and Franco Borruto radiographs relates to the fact that calcium may occur, not only as calcium phosphate [Ca3(PO4)2], but also as calcium oxalate (CaC2O4), the latter not being stained by hematoxylin and eosin (H&E) but readily detected by its birefringent nature in polarized light. Detailed correlation of serial thin slices of the specimen with radiographic features is largely an educational exercise, but is the most time-consuming step in the examination of the non-palpable breast lesion [97]. FNA cytology (FNAC) for breast tumors was first described and performed in 1930. Thirty years later, it gained acceptance first in Europe and about a decade later in North America. Since 1970, FNAC has grown in popularity and is now routinely used in the initial diagnosis of palpable breast masses in the USA and in other parts of the world. Fast staining methods of the aspirate enables reporting within ten minutes of the aspirate being performed. Training and experience is important in obtaining satisfactory smears for diagnosis, and pitfalls are false-negative and false-positive findings, which may have dire consequences for the patient if cytological diagnosis is the final arbiter, and raise questions regarding the diagnostic utility of FNA as a replacement for open biopsy in many clinical situations. False-positive diagnoses may result from atypical epithelial proliferations, fibroadenomas, or inflammatory lesions. False-negative aspirates may occur because of technical errors, cystic lesions, and under-diagnosis of low-grade neoplasms. Conditions such as benign mammary dysplasia and sclerosing adenosis are the most common sources of highly cellular smears and often show marked atypia, which makes distinction from carcinoma difficult. In addition, atypical papillary formations present a diagnostic problem, and biopsy is indicated to exclude a papillary carcinoma. FNA very seldom cause traumatic complications, and these are usually of a minor degree. Seeding along the needle track has occurred, but in most cases with a largercaliber (18-gauge) needle. Aspiration itself has been shown to have no effect on the survival rates in breast carcinoma. Contemporary reports show that around 90% of cases of breast cancer can be detected with confidence by means of this procedure. The reduction in scar formation facilitates future evaluation of the patient as scar tissue often interferes with the interpretation of mammograms. Cost-effectiveness is evident in terms of decreased use of anesthetics and operating time and a reduction in the use of frozen section histology by about 80% [98]. The exact role of FNA in the diagnosis of palpable breast lesions is still uncertain. The triple-diagnosis protocol has been suggested as a replacement for open biopsy of palpable breast masses in many clinical situations. Following this algorithm, the results of palpation, mammography, and cytology are combined to guide management. Mammography should precede FNA or follow the cytological procedure by two or more weeks. Patients with a positive triple diagnosis should undergo open biopsy or confirmatory intraoperative frozen section. Positive FNA results would be useful for preoperative counseling as well as serving as a diagnostic procedure for clinically suspicious lesions in patients wishing a confirmatory test before Breast Biopsy 23 open biopsy is performed. Patients with discordant triplet results should be referred for open biopsy. The management of patients with negative triplet results is less clear. From the available data, it appears that approximately 2% of patients with negative triplet results have carcinoma. Based on these results, we cannot recommend replacing open biopsy by the triple diagnosis method in most patients with a persistent dominant mass. In most cases, a biopsy is indicated. Surgeons who plan to follow a breast mass with clinical examination may be able to reduce their false-negative rate by performing FNA [99]. Breast FNA has a reported mean specificity of 99%, and a reported sensitivity of 70-99%. The false-positive rate varies from 0-0.4% in most larger series, with a reported false-negative rate ranging from 0.7-22%: however, higher false-negative rates have been reported in tumors under 2 centimeters (cm) in diameter. The FNA technique uses a fine, 20-gauge or less, needle and is not associated with a significant risk of tumor growing out the needle tract. FNAC is not effectively used if a breast mass cannot be palpated or distinguished from fibrous tissue within the breast. The procedure can be applied to nonpalpable masses detected by mammography by employing stereotactic techniques. The cytological samples obtained from FNA can be used to distinguish atypical ductal hyperplasia from DCIS or invasive ductal carcinoma. However, cytological criteria to effectively distinguish DCIS from invasive adenocarcinoma are not definitive in many cases, and are dependent on variables related to the type of intraductal tumor, the size and character of the cell groups, and the presence of single or disaggregated tumor cells. Employing current cytological criteria, LCIS may be distinguished from invasive lobular carcinoma in some cases: however, the individual LCIS cells are not morphologically distinct from lobular carcinoma cells. Atypical lobular hyperplasia has cellular features essentially the same as those seen in LCIS. FNAB employs larger needles of 14-16gauge, does not require surgery, and uses only a small amount of material [100]. FNAB can also be used to acquire material for special studies. This is especially useful with small tumors (≤ 1 cm) when most of the material is needed to make a histological diagnosis. Immune-staining techniques can be used on FNAB to investigate proliferation by bromodeoxyuridine (BrdU) uptake or Kiel-67 (Ki-67) labeling. Immune-staining techniques can also be used to identify oncoprotein expression, such as of tumor protein 53 (p53). Fluorescence in situ hybridization (FISH) is a technique that can be used to gather cytogenetic information directly from interphase tumor cells and is well suited for use with FNAB material because the harvested nuclei are intact and no cumbersome dissociation processing is needed. Flow cytometric techniques can be applied to FNAB material to study deoxy-ribonucleic acid (DNA) content and synthesis (S)-phase fraction. Material acquired by FNAB can also be analyzed by the polymerase chain reaction (PCR) followed by mutation detection. It is essential that the FNAB harvest is representative, ample, and well prepared for the success of these studies [101]. 24 Ciro Comparetto and Franco Borruto Recently, there has been a considerable increase in the use of both FNAB (aspiration cytology) and breast tissue-CNB. In patients with suspected breast cancer, needle biopsy is frequently used to confirm the diagnosis before treatment is planned. This allows a more thoughtful approach to the patient and full screening for possible metastatic disease prior to definitive surgery. Needle biopsy techniques are simple, rapid, can be performed in the doctor’s office, and save time, equipment, and hospital beds. Complications are few. Aspiration cytology has the advantage that it is quick to perform, the preparation can be examined almost immediately, and, in the event of an unsatisfactory smear, the procedure can be repeated. However, the diagnosis is based on purely cytological evaluation, and the information obtained is somewhat limited. Reported accuracy rates range from 42-96%. False-positive reports are rare but have occurred in most centers, and a high degree of accuracy will only be obtained by experienced practitioners. TissueCNB has the advantage that the diagnosis is based on histopathological assessment, but the procedure is slightly more time-consuming, is more traumatic for the patient, and the equipment is more expensive. Accuracy rates range from 67-98.5%. Studies comparing the use of FNAC and tissue-CNB in the diagnosis of mammary carcinoma have produced variable results. Both methods have advantages and disadvantages, and the choice of technique must depend on the clinical situation and the preferences and skills of the practitioners involved in the management of the patient [102]. In developed countries, in the last 20 years, mammographic screening programs, which have been used extensively, are designed to detect the earliest possible breast cancer. The FNAC report is extremely important because it gives the necessary information for the management of patients, to proceed with more invasive diagnostic methods or surgical treatment, and to decide what kind of operation to perform. In the preoperative phase, FNAC has taken a fundamental role of both palpable and nonpalpable lesions, using ultrasound or stereotactic guidance. New developed techniques, breast biopsy instrumentation (ABBI) and Mammotome (Devicor Medical Products, Inc., Cincinnati, OH, USA) have the advantage of complete removal of breast lesions, but this is not possible in all the examined cases. In developing countries, economical restrictions and low budget for health care and screening programs put the patients at a disadvantage because of the prohibitive cost of sophisticated diagnostic methods, thus it is recommended that FNAC be used as a routine diagnostic method because of its low cost compared with the others and this policy maximizes the availability of health care to women with breast cancer. FNAC plays an important and essential role in the management of patients with breast lesions and offers a great potential for prediction of patient outcome, disease response to therapy, and assessment of risk of developing breast cancer. The reliability and efficiency of the method depends on the quality of the samples and the experience of the medical staff that performs the aspiration [103]. However, the success of breast FNA is highly dependent on the adequate preparation of Breast Biopsy 25 cytological conventional smears. The liquid-based cytology (LBC) technique consists of an automated method for preparing thin-layer cytological samples from cell suspensions collected in alcohol-based preservative. LBC is designed to improve conventional smears by avoiding limiting factors such as obscuring material, air-drying, and smearing artifacts. LBC preparations of breast aspirates demonstrated better cellular preservation, less cell overlapping, and elimination of blood and excessive inflammation compared to conventional smears. Conversely, alterations in architecture and cell morphology as well as loss of myoepithelial cells and stromal elements have been described in LBC specimens, requiring training before applying this technique for diagnosis. Studies have shown a similar accuracy between LBC and conventional smears for the diagnosis of breast lesions. LBC also permits the use of residual material for ancillary tests, which is an important advantage compared to conventional smears. LBC can be safely applied to breast FNA, showing a similar diagnostic accuracy to conventional smears [104]. CNB is increasingly being used in the investigation of breast disease whether this is asymptomatic and suspected after screening mammography, or presents symptomatically in the patient. In most cases, the result of the procedure provides a definitive diagnosis or at least provides information that is used to plan the further management of the patient. There are, however, many unresolved issues with the use of CNB: for example, with regard to the amount of information that can be reliably derived from CNB or with regard to the management of the patient after some CNB diagnoses. ER and progesterone receptors (PR) and human epidermal receptor 2 (HER-2) are reported on both CNB and excisional specimens, but there continues to be debate over which represents the more appropriate specimen type on which to perform these tests. There are many possible diagnoses after CNB for which the management is not straightforward and around which there may be controversy, or just a lack of sufficient evidence to support a definite management plan. These “lesions of uncertain malignant potential” include papillary lesions, fibroepithelial lesions with cellular stroma, mucocoele-like lesions, and radial scars. Currently, these are removed surgically but there may be an argument for more conservative management including vacuum-assisted core biopsy (VACB) in some cases [105]. CNB is an established alternative to surgical biopsy, and CNB can avoid excess surgical biopsies in many patients. In addition to accurate histological diagnosis, there is interest in obtaining prognostic information from CNB, especially for patients being considered for preoperative (neoadjuvant) therapy. CNB provides useful information about histological type and grade. However, an unavoidable problem of CNB is underestimation of invasion. Several aspects of CNB remains controversial, such as diagnosing papillary lesions by CNB, problems regarding tumor cell displacement after CNB, and management of lobular neoplasia on CNB [106]. In the last decade, percutaneous breast biopsies have become a standard for the management of breast diseases. Biopsy clips allow for precise lesion localization, thus 26 Ciro Comparetto and Franco Borruto minimizing the volume of breast to be resected at the time of surgery. With the development of many imaging techniques (including mammography, sonography, and breast MRI), one of the challenges of the multidisciplinary team became to synthesize all informations obtained from the various imaging procedures. The use of biopsy markers after percutaneous biopsy is one of the keys for optimal patient management, helping the radiologist to deal with multiple lesions, to insure correlation across different imaging modalities and to follow-up benign lesions, helping the oncologist by marking a tumor prior to neoadjuvant chemotherapy, helping the surgeon by facilitating preoperative needle localization, to precisely mark the margins of extensive disease and to guide intraoperative tumor resection, and helping the pathologist to insure the lesion of interest has been removed and to identify the region of interest in a mastectomy specimen. We believe biopsy clip markers should be deployed after all percutaneous interventions [107]. FNAB and CNB techniques require training and cytopathologist support. In contrast, breast cyst aspiration using a 21- or 22-gauge needle is a simple, cost-effective, and minimally invasive procedure. The technique is easy to learn and can be practiced on a breast model. Breast cyst aspiration may be attempted in many women who present with a palpable, dominant breast mass. If clear fluid is aspirated and the mass resolves, malignancy is unlikely, and breast cyst is the probable diagnosis. In this situation, reevaluation in four to six weeks is appropriate: if the cyst has not recurred, only routine mammographic surveillance is required. Referral for FNAB or excisional biopsy is indicated if the aspirate is bloody or extremely tenacious, if no fluid can be aspirated, or if there is residual mass after aspiration. Complications such as local discomfort, bruising, and infection are uncommon [108]. Established methods of breast cancer detection have well-described limitations, and new diagnostic techniques are evolving continually to improve diagnostic accuracy. Studies to date suggest the presence of 5-12 independent ductal lobular systems per breast, each harboring complex cellular fluids contributed by local and systemic processes. The intraductal approach to breast cancer has been invigorated in the last decade by a series of papers exploring ductal-based screening through nipple aspiration and lavage and ductal exploration through endoscopy. The merging of these efforts to define the earliest biological changes in the progression toward breast cancer is opening new fields for both bench-translational and clinical research. These techniques have already begun to show value in defining the presence and extent of proliferative disease in high-risk patients, allowing for more informed therapeutic decision making [109]. Ductal lavage is a recently introduced technique for non-surgical breast epithelial sampling of asymptomatic high-risk women. It is based on exfoliative cytology of duct epithelial cells, obtained by cannulation and lavage of ducts that produce nipple fluid after breast massage and nipple aspiration. The technique is performed with topical Breast Biopsy 27 anesthesia and involves cannulation of any fluid-yielding nipple orifice with a speciallydesigned catheter for lavage and aspiration of the ductal system. Ductal lavage provides information like that obtained by cytological examination of nipple aspiration fluid and random periareolar FNA. Women who demonstrate cytologic atypia on these tests can be assumed to be at higher risk for breast cancer and may benefit from prophylactic medication. Ductal lavage promises to provide more reproducible sampling of the same area of the breast, as opposed to methods of random sampling, such as random FNA, but the degree of reproducibility remains to be demonstrated. If reproducibility is greater than with random periareolar FNA, ductal lavage may represent an important advance in the design of phase II chemoprevention trials, particularly because it also provides another source of material for judging response to prevention agents (i.e., the protein component of nipple aspiration fluid), which can be analyzed for levels of hormones, specific proteins, and protein profiles obtained with proteomics [110, 111]. Ductal lavage and cytological analysis of the effluent is a more sensitive and specific test than nipple aspiration cytological analysis and random FNA. The dilemma lies in what should be done after a ductal lavage yielding malignant or atypical cells [112]. The ductal approach to breast cancer allows assessment of breast ductal epithelial cells and their local microenvironment in a graded process of increasing invasiveness. Samples of ductal epithelial cells sufficient for cytological diagnosis may be safely collected, titers of individual proteins showing variation with breast cancer status may be measured, and abnormal pathology within the breast ducts may be directly visualized. Identification of surrogate molecular markers may facilitate early breast cancer detection, in conjunction with cytological assessment, and be useful for individual prediction of breast cancer risk and assessment of treatment response. The small quantities of nipple aspiration fluid available for analysis, and difficulties in identifying and cannulating ducts remain important limitations of these techniques [113-116]. To improve disease monitoring over time and to avoid painful procedure such as tissue biopsy, liquid biopsy may represent a new precious tool. Indeed, it represents a basin of “new generation” biomarkers that are spread into the bloodstream from both primary and metastatic sites. Moreover, elevated concentrations of circulating tumor DNA (ctDNA) as well as circulating tumor cells (CTC) have been found in blood plasma of patients with various tumor types. Nowadays, several innovative approaches have been introduced for the detection and characterization of CTC and ctDNA, allowing a real-time monitoring of tumor evolution [117, 118]. Today, radiology is an essential step in the pathological analysis of breast biopsies. It is determinant at each stage of the management of non-palpable lesions, clusters of microcalcifications, and opacities, whether this concerns the needle biopsy or the surgical excision. Firstly, an X-ray is necessary to ensure that the biopsy specimen has been adequately sampled and when samples with microcalcifications are selected by the 28 Ciro Comparetto and Franco Borruto radiologist, management can be more specific and accurate. In the case of surgical specimens, the X-ray confirms the presence of the radiographic abnormality or the clip indicating the site of the surgical excision which guides sampling. Some radiographic features also provide information on underlying pathologies allowing management to be adapted accordingly. Radiographs are also important to ensure that microscopically detected microcalcifications or lesions exactly correspond to the radiographic abnormality in size and location. The paraffin block can also be X-rayed to select those containing microcalcifications for additional slicing. It is also important to identify the presence of modifications caused by the biopsy (fibrosis, hemorrhage, and inflammation) and to carefully recognize displacement of epithelial cells and pseudoemboli resulting from the needle procedure. Such correlation between radiology and pathology is essential so that appropriate management of the specimen can be adapted and to avoid pitfalls arising from preoperative procedures [119]. With the ability to perform good diagnostic mammography, breast ultrasound, ductography, cyst aspiration, abscess drainage, and stereotactic or ultrasound-guided percutaneous biopsy, the modern breast radiologist should play the central role in breast diagnosis. The definitive diagnoses afforded by percutaneous breast biopsy that obviate surgery entirely in benign lesions and streamline the therapeutic surgery for malignant lesions now can be obtained routinely. The future holds even more exciting challenges for the radiologist as percutaneous lumpectomy becomes a reality. Thus, the breast radiologist, armed with the technology and techniques of the 21st century, truly stands on the threshold of a new era [120]. In summary, investigation of women with a breast lump or suspicious change in breast texture starts with a history, physical examination, and usually mammography. The clinical history should establish how long the lump has been noted, whether any change has been observed, and whether there is a history of biopsy or breast cancer. Risk factors for breast cancer should be noted, but their presence or absence should not influence the decision to investigate a lump further. The breast physical examination should aim to identify those features that distinguish malignant from benign lumps. Mammography can often clarify the nature of the lump and detect clinically occult lesions in either breast. FNA can establish whether the lump is solid or cystic. When a tumor is solid, cells can be obtained for cytological examination. Ultrasonography is an alternative method to FNA for distinguishing a cyst from a solid tumor. Whenever reasonable doubt remains as to whether a lump is benign or malignant, a biopsy should be carried out. When surgical biopsy is used, the aim is to remove the whole lump in one piece along with a surrounding cuff of normal tissue. CNB, either clinically or imagingguided, can usually establish or exclude malignancy, thus reducing the need for surgical biopsy. Thermography and light scanning are not recommended diagnostic procedures. The value of MRI is progressly growing. The choice of procedure should consider the Breast Biopsy 29 experience of the diagnostician and availability of the technology in question. The workup should be completed expeditiously and the patient kept fully informed throughout. Even when malignancy is not found, it may be prudent, in some cases, to arrange followup surveillance [121]. For the evaluation of non-palpable breast lesions, imaging-guided large-CNB are increasingly replacing needle-localized open breast biopsies. The sensitivity rate of large-CNB for the diagnosis of breast cancer is high (97%). The reclassified agreement rate between CNB and subsequent surgical biopsy or long-term mammographic follow-up is also high (94%). In case of 20% breast cancer prevalence among women referred after screening (as in the USA), the risk of breast cancer despite benign large-CNB result is less than 1%. In European countries, however, prevalence of breast cancer among referred women is 60-70%. This would result in a risk of breast cancer despite benign large-CNB result of 4-6%. The imaging-guided large-CNB is a promising alternative for the needle-localized breast biopsy. However, additional research is needed to explore the limiting factors of the technique. Without such detailed knowledge, a benign histological diagnosis on large-CNB in countries with high prevalence of malignancy among referred women should be interpreted with caution [122]. Most countries have now adopted a triple assessment approach, i.e., clinical, imaging, and pathology, to breast diagnosis, with FNAC as the first-line pathological investigation in both screening and symptomatic populations, except for microcalcifications. Pathologists specialized in cytopathology are best qualified to collect and interpret FNAC samples, but this is not always possible or practical. Radiologists involved in breast imaging should ensure that they have the necessary skills to carry out FNAC under all forms of image guidance. Best results are achieved by a combination of both techniques, as shown in the imaging-guided FNAC in the presence of the cytopathologist. Most of European countries use similar reporting systems for breast FNAC, in keeping with European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis, although some still prefer descriptive reporting only. When triple assessment is concordant, final treatment may proceed based on FNAC, without a tissue biopsy. ER and PR assessment can be done safely on FNAC material. However, not all institutions may have expertise in doing this. HER-2 protein expression on direct cytological preparations is insufficiently reliable for clinical use, although its use for FISH is possible, if expertise is available. Most clinicians practise a degree of one-step diagnosis with a cytopathologist present in the out-patient clinic. Formal recognition of the importance of the time spent outside the laboratory, both for cytopathologist and cytotechnologist, is necessary to ensure appropriate resourcing. The use of CNB has increased, although not always for evidence-based reasons. CNB and FNAC are not mutually exclusive. FNAC should be used in diagnosis of benign, symptomatic lesions and CNB in microcalcifications, suspicious FNAC findings, and malignancies where radiology cannot guarantee stromal invasion [123-136]. Chapter III Benign Breast Diseases Benign breast diseases can be divided into congenital, developmental, inflammatory, neoplastic, “fibrocystic,” and miscellaneous categories [137]. The relevant medical literature does not reflect the entire spectrum of benign breast diseases or the appropriate treatment. Most textbooks on breast disease emphasize breast cancer and the late manifestations of benign breast disease that often require surgical treatment. More than 180,000 cases of breast cancer occur each year in the USA. The number of women with benign breast disease is far greater and can be counted in the millions. For these patients, the well-trained primary care physician can provide appropriate evaluation and treatment, including recommendations for referral. For most patients with breast symptomatology, the goal is relief of symptoms and resolution of the problem. To accomplish this requires a contemporary knowledgebase combined with adequate time spent with the patient [138]. Thus, breast health means more than breast cancer. At least 50% of patients seen at a multidisciplinary breast center have benign conditions [139]. The most common breast problems for which women consult a physician are breast pain, nipple discharge, and a palpable mass. Most women with these complaints have benign breast disease. Breast pain alone is rarely a presenting symptom of cancer, and imaging studies should be reserved for use in women who fall within usual screening guidelines. A nipple discharge can be characterized as physiological or pathological based on the findings of the history and physical examination. A pathological discharge is an indication for terminal duct excision. A dominant breast mass requires histological diagnosis. A breast cyst can be diagnosed and treated by aspiration. The management of a solid mass depends on the degree of clinical suspicion and the patient’s age [140]. When faced with a suspicious clinical or mammographic finding, patients may be anxious about the possibility of breast cancer. Because many of these findings are 32 Ciro Comparetto and Franco Borruto benign, clinicians attempt to make a rapid and accurate diagnosis to definitively rule out carcinoma. Imaging plays a key role in diagnostic strategies [141]. Benign breast disorders, classified by the aberrations of normal development and involution (ANDI) system, constitute the major workload in breast clinics (Table 2). Breast pain (mastalgia) is classified as cyclical, and non-cyclical extra-mammary causes such as ribircage pain must be identified. Most patients need reassurance alone, but those with moderate/severe pain present for more than six months may need treatment: RCT have shown danazol, bromocriptine, and TMX to be effective. Fibroadenoma is the commonest benign solid lump in women aged 15-30 years. The diagnosis must be confirmed by triple assessment. Cysts occur usually in women of middle to late reproductive life. After ultrasound has confirmed the lump as cystic, it can be aspirated. Nipple discharge should be tested for the presence of hemoglobin (Hb). Those with Hb+ discharge may require microdochectomy for treatment and diagnosis, common causes being duct papilloma and duct ectasia. Breast abscesses may occur during lactation or in women with duct ectasia and are treated by incision or aspiration together with antibiotics [142]. Benign breast disorders can be considered from four points of view: clinical presentations, clinical significance, management, and pathogenesis. Understanding the pathogenesis is important for rational management and for assessing clinical significance. Clearly understood nomenclature is also important. Clinicians have tended to concentrate on the condition usually known as fibrocystic disease, a clinical condition (painful nodularity), but a terminology which relates to a histological picture of fibrosis, cyst formation, and epithelial hyperplasia, now known to occur in both asymptomatic and symptomatic breasts. To address these problems, the ANDI concept has been proposed as a framework for benign breast disorders which is comprehensive, accurate in terminology, and based on pathogenesis. For each disorder, there is a spectrum from normal through mild abnormality (aberration) and in some cases (only) to disease. This concept encompasses pathogenesis, clinical and histological significance, and general principles of management. It has proved particularly useful in giving an understanding of benign breast disorders to doctors in training [143]. Several large-scale prospective RCT establish the validity of managing probably benign breast lesions with periodic mammographic surveillance as a safe and effective alternative to immediate tissue diagnosis. This approach to mammographic interpretation is now very widely accepted: the American College of Radiology (ACR) indeed includes “probably benign – short interval follow-up suggested” as one of the five final assessment categories in the BI-RADS, which all American radiologists are encouraged to use. There is also consensus that probably benign interpretations should involve: 1) cases restricted to non-palpable lesions; 2) use of the specific imaging criteria described in the prospective RCT; and Benign Breast Diseases 33 3) preinterpretation comparison with prior mammograms, if available, to ensure that new or progressing lesions undergo prompt biopsy (it makes no sense to recommend follow-up for a lesion that has already demonstrated interval progression when the very demonstration of progression during surveillance is what prompts biopsy instead of continued follow-up). Table 2. Aberrations of normal development and involution (ANDI) system classification (adapted from radiopaedia.org, 2017) Disorders of development: polymastia and polythelia; accessory axillary breast tissue; congenital nipple retraction; macromastia; fibroadenoma; phyllodes tumor; adolescent hypertrophy. Disorders of cyclical change: mastalgia; nodularity. Disorders of involution: fibrocystic change: o cysts; o fibrosis; o sclerosing adenosis. periductal mastitis. Yet, unresolved issues concerning probably benign lesions include whether initial full problem-solving imaging should be performed in all cases, whether to use patient age and lesion size (for solitary masses) as additional criteria in deciding between the management alternatives of mammographic follow-up and immediate tissue diagnosis, and what should be the specific timing, frequency, and duration of the follow-up examinations that constitute the surveillance protocol [144]. The discussion of abnormal results of breast imaging and abnormal pathological findings can be challenging for health care professionals and often is stressful for patients. Although most imaging findings and biopsy results are negative and do not infer a substantial increase in breast cancer risk, the subsequent conversation between the patient and her practitioner is more effective and informative with a thorough review of the pathological results and an appreciation of the importance of radiological-histological concordance [145]. The strategies of treating breast benign conditions depend upon the need to relieve symptoms, 34 Ciro Comparetto and Franco Borruto to prevent recurrence, and to guard against malignancy which vary according to the diagnosis. Morbidity also must be prevented [146, 147]. Disorders of the female breast in the pediatric age group are a relatively common finding: however, there is limited information in the current literature. The plastic surgeon treating these patients is faced with a wide range of reconstructive problems. By understanding the various breast disorders, the plastic surgeon can better diagnose and treat this patient population appropriately [148]. Pediatric chest wall and breast deformities present as a wide spectrum of anomalies, and often occur coincidentally. Chest wall abnormalities fall into two categories: congenital (which are largely hypoplastic) and deformational (including both chest wall malignancies and postoperative abnormalities). Breast abnormalities can be categorized into three groups, including hypoplastic, hyperplastic, and deformational anomalies. Hypoplastic breast anomalies require reconstruction with augmentation techniques and are often associated with significant reoperative rates, as are deformational anomalies. Hyperplastic abnormalities require reduction techniques and are less likely to require re-operation. Considerations about surgical correction of pediatric chest wall and breast deformities often require coordinated efforts between pediatricians and pediatric plastic surgeons with anticipation of continued growth of the child and careful timing for treatment to maximize functional and aesthetic outcomes [149, 150]. Breast lesions, including mastalgia, benign proliferative changes, and benign masses, including fibroadenomas, are common in adolescents and young adult women. Breast cancer is rare in women less than 20 years old and uncommon in women less than 30 years old. Discrete masses in women less than 30 years old that do not feel suspicious on examination can be observed for one or two months. If they persist, then a FNA can differentiate those that are benign and can be observed versus those that need either an excisional biopsy or definitive surgery. Mammography has little role in the diagnosis of women less than 30 years old except in those individuals with highly suspicious lesions on examination [151]. So, though females may develop breast masses early in life, the risk of malignancy is extremely low. Therefore, most breast masses in the young can be managed conservatively without surgery. Breast masses in young girls may represent thelarche or tumors of adjacent structures, but are unlikely to be malignant. The most common form of bilateral breast enlargement in prepubertal girls is premature thelarche, a benign, transient, and incomplete form of precocious puberty. Fibroadenoma is the most common cause of breast mass in female adolescents. In the rare case in which a breast mass in this population is malignant, it is more likely to be a non-carcinomatous or metastatic cancer. Because of the low risk of malignancy and the relatively different composition of the adolescent as compared to the adult breast, mammography is not recommended for routine screening or routine imaging of breast masses in adolescents. The role of breast self-examination should be further studied [152]. Anyway, although Benign Breast Diseases 35 99% of breast lesions in female adolescents are benign tumors, surgical intervention is commonly required [153]. Breast disease in the adolescent female encompasses an expansive array of topics. Benign disease overwhelmingly dominates the differential diagnosis and dictates a different protocol for care in the adolescent compared with the adult patient to avoid inappropriately high assessments of risk and unnecessary diagnostic procedures and surgery. There are also emerging issues pertaining to the care of the adolescent breast, such as breast augmentation, nipple piercing, and management of the adolescent patient with a family history of breast cancer [154, 155]. Macromastia is a rare psychologically and physically disabling condition of abnormal breast tissue enlargement more than the normal proportion characterized by excessive breast growth. The condition may be caused by glandular hypertrophy, excessive fatty tissue, or combination of both. The usual physiological enlargement of female breast occurs over three to five years and the female breast size is related to body habitus and hereditary characteristics. The etiology of macromastia is usually undetermined, however, hormonal excesses and hypersensitivity of the target organ have been found in some cases. It is safe to surmise that the pathological condition is multifactorial, with both inherited and acquired aspects. Cases of hyperprolactinemia have been reported by some workers. Immunological risk factors for development of macromastia have also been seen in some groups of patients with myasthenia gravis, chronic arthritis, and Hashimoto thyroiditis. Ultrasonography may show no breast parenchyma abnormalities, while mammography may be indicated in some patients who are 40 years or older. Hormonal assay can be done but its value in treatment is doubtful. Drugs are only marginally effective in reversing gigantomastia: therefore, surgery remains the mainstay of treatment. Management of macromastia can be physically, socially, and psychologically satisfying to both the patients and the surgeons [156]. To date, there is no universal classification or accepted definition for this condition. Many authors cite gigantomastia as breast enlargement that requires reduction of over 1500 grams (g) per breast. However, there is discordance in the literature with the weight of reduction ranging from 0.8-2 kilograms (kg). Practically, this is a postoperative definition which is of little use to the clinician in terms of patient management or prognosis [157]. Macromastia is a deforming, disabling, and painful condition, especially in the adolescent. Multiple procedures have been advocated and are successful for the reduction of breast tissue. In addition, adjunctive therapy with hormones may prevent relapse. In the various techniques for reduction, it is important to have a clear understanding of breast vascular and neural innervation, to maintain maximum security in reduction without loss of excessive vital tissue. Although both sensory ability and lactation function are diminished with most procedures and eliminated with some, careful planning and patient counseling in all cases should lead to maximal benefit and optimal results [158]. Currently, there are no patient- or disease-oriented evidence-based 36 Ciro Comparetto and Franco Borruto guidelines for the treatment of this condition. A significant relationship was found (p < 0.01), as was an odds ratio (OR) of 7.0, for the likelihood of recurrence using a reduction mammoplasty as opposed to a mastectomy. Based on the evidence, certain interventions are more effective for the treatment of virginal mammary hypertrophy. On diagnosis of virginal mammary hypertrophy, TMX therapy may be considered based on previous literature, barring any medical contraindications. A subcutaneous mastectomy with complete removal of breast tissue is the procedure least likely to lead to recurrence but is more deforming. Reduction mammoplasty gives an improved aesthetic breast, but it is important to counsel the patient on the likelihood of increased recurrence. TMX therapy following surgery may decrease the recurrence rate [159]. Pain is one of the most common breast symptoms experienced by women. It can be severe enough to interfere with usual daily activities, but the etiology and optimal treatment remain undefined. Breast pain (mastalgia) is the commonest breast symptom presenting to general practitioners and breast surgeons alike. To make a full assessment of the cause, all patients require a full history, physical examination and, sometimes, radiological investigations. Diary cards are often helpful. Breast pain is typically approached according to its classification as cyclical mastalgia, non-cyclical mastalgia, and extramammary (non-breast) pain. The commonest cause is cyclical mastalgia. Cyclical mastalgia is breast pain that has a clear relationship to the menstrual cycle. Noncyclical mastalgia may be constant or intermittent but is not associated with the menstrual cycle and often occurs after menopause. Extramammary pain arises from the chest wall or other sources and is interpreted as having a cause within the breast. The risk of cancer in a woman presenting with breast pain as her only symptom is extremely low. Mastalgia is a common cause of anxiety among women and frequently leads to a primary care clinic for consultation. Fortunately, mild premenstrual breast discomfort lasting for one to four days can be considered “normal.” However, moderate-to-severe breast pain lasting over five days can interfere with usual activities, lead to unnecessary medical tests, and potentially invite the use of ineffective, occasionally harmful medications. Despite the severity of some patients’ symptoms, mastalgia is still considered a trivial complaint by many physicians: often, it is felt to be psychological in nature. After appropriate clinical evaluation, most patients with breast pain respond favorably to a combination of reassurance and non-pharmacological measures. For the remainder 15% of mastalgia patients who have life-altering pain and still request treatment, simple lifestyle changes should be suggested initially, such as well-fitting wearing, weight reduction, regular exercise, a decrease in dietary fat intake, a reduction in caffeine intake, and discontinuance of oral contraceptives or hormone replacement therapy (HRT). Unfortunately, there is a paucity of evidence for the usefulness of these measures. In a few patients, however, mastalgia is severe enough to deserve further evaluation and treatment. Overall, 92% of patients with cyclical mastalgia and 64% with Benign Breast Diseases 37 non-cyclical mastalgia can obtain relief of their pain with the judicious use of several available therapies. If pain is persistent or severe, a variety of pharmacological agents exist. The most effective with least side effects is a three-six-month course of low-dose TMX [10 milligrams (mg)]. Other proven agents include evening primrose oil supplements, danazol, bromocriptine, or GnRH analogues, but these have a higher sideeffect profile and are rarely indicated nowadays. The potentially serious adverse effects of these medications limit their use to selected patients with severe, sustained breast pain. Newer treatments include lisuride maleate and topical non-steroidal antiinflammatory (NSAI) preparations [160-162]. Predicting which treatment will be most useful for any woman may be challenging. No differences in success rates were found to be associated with factors such as reproductive history, presenting complaint, personal or family history of breast disease, or subsequent need for breast surgery. The peak (but not basal) serum prolactin (PRL) levels in response to thyrotropin releasing hormone (TRH) stimulus has been predictive of success for hormonal treatment but is relatively invasive. The prognosis for women with breast pain is not always predictable. Women with cyclical breast pain often are relieved by events that alter their hormonal milieu, whereas non-cyclical breast pain may last only one to two years [163]. Differentiation into cyclical and non-cyclical patterns on a simple pain chart is useful for objective assessment of pain severity and for selection of appropriate drug therapy and subsequent monitoring of response. About 85% of new patients will be satisfied with adequate reassurance, but some 15% will have persistent pain and warrant medical treatment. No ideal agent exists, and the choice of drug will depend on efficacy, side effects, and cost. Non-cyclical pain has a lower response rate compared to cyclical mastalgia, but differentiation of a subgroup with chest wall pain leads to an overall 90% response to treatment by local infiltration with steroids and lignocaine. Newer agents such as luteinizing hormone-RH (LHRH) agonists are currently undergoing evaluation in doubleblind RCT against placebo. The management of nodularity is based on the clinical differentiation of the normal spectrum of physiological change within the breast (ANDI), requiring simple reassurance, from a true dominant breast nodule that will require excisional biopsy to exclude malignancy. When pain and lumpiness coexist, some reduction in overall nodularity (with the use of agents given for mastalgia) may occur [164]. Congenital breast and chest wall anomalies represent a relatively common set of disorders frequently encountered by breast and pediatric plastic surgeons with a spectrum of severity that ranges widely from the relatively benign polythelia to the very complex disorders such as Poland’s syndrome and tuberous breast deformities. While the former can be treated in a single surgical setting with minimal morbidity, the more complicated disorders often require a staged reconstructive algorithm. The treatment for many of these conditions includes surgical correction. If the child is still growing, 38 Ciro Comparetto and Franco Borruto treatment timing is crucial and many of the surgical corrective procedures require more than one operation over many years. Some disorders also require a multidisciplinary management for both workup and treatment [165]. Although these deformities have less impact on functional capacity, the psychological consequences can be serious in adolescent patients. They can experience embarrassment, social isolation, and complexities during sexual development, and this results in problems with relationships [166]. Poland’s syndrome is characterized by hypoplasia or absence of the breast or nipple, hypoplasia of subcutaneous tissue, absence of the costosternal portion of the pectoralis major muscle, absence of the pectoralis minor muscle, and absence of costal cartilages or ribs 2, 3, and 4 or 3, 4, and 5. The chest wall defect is often associated with a lung hernia. Clinical manifestations are extremely variable and rarely are all the features recognized in one individual. Fortunately, it is invariably unilateral, allowing for an easier reconstruction. Single-stage reconstruction of the chest wall combined with simultaneous augmentation mammoplasty and transfer of an island pedicle myocutaneous flap of latissimus dorsi muscle are major improvements over previous multiple-stage procedures that provide less satisfactory cosmetic results in management of patients with Poland’s syndrome [167]. The main purpose of surgical correction in Poland’s syndrome is to improve chest wall symmetry and correct breast hypoplasia. Creation of an anterior axillary fold and smoothing out the infraclavicular defect greatly improves the result. Cardiorespiratory function may be impaired, but serious conditions requiring early operative correction are rare. When present, unilateral costochondral agenesis involves one to three segments in the mid-anterior chest and sternal depression to that side. Operative planning in such cases includes a multilayered approach to provide a solid base for soft tissue reconstruction of the more superficial layers [168]. The presence of accessory breast tissue such as extra nipples (polythelia) and extra breast (polymastia) is relatively common, with a high incidence of being misdiagnosed in clinical medicine. Although polythelia is congenital in origin and is identifiable at childhood, polymastia may not be evident until the influence of sex hormones during puberty [169]. The common congenital anomalies of the nipple include inversion, clefts, and supernumerary nipples. Supernumerary nipples are common anomalies, and their significance is usually limited to cosmetic concerns. However, a high index of suspicion should be maintained during physical examinations, because any disease process that involves anatomically normal breasts may affect aberrantly located breasts or nipples as well. These anomalies may be associated with several systemic disorders, particularly urinary tract abnormalities [170, 171]. Several different techniques have been developed and currently are in use for correction of the inverted nipple. The diversity of techniques indicates the lack of a good, sustainable, and durable solution for this quite widespread problem. In a new technique, two flaps are inserted beneath the nipple through a small Benign Breast Diseases 39 tunnel. The advantages of this procedure are its simplicity, the creation of a durable support for the nipple, and the lack of transverse scars in the areola surrounding the nipple. The follow-up period up to four years demonstrates the validity of this technique [172]. The tuberous breast classification proposed by Grolleau does not account for a minor form of the deformity characterized by isolated NAC protrusion with a normal breast base. The breast is subjected to hormonal influences as early as the prepubertal period. These influences result in thrusting forces with both horizontal (estrogen) and vertical (progesterone) vectors, unfortunately not always balanced and harmonious. Close observation of the anomaly substantiates the basic anatomical defect, namely, the structural congenital dermal weakness of the NAC already described in all forms of tuberous breast deformity. This weakness explains the morphological anomaly and confirms that all types of tuberous breast deformity constitute a spectrum of a single entity. It indicates also that the tuberous breasts classification should include, in addition to the three types (types I-III) already described, a fourth type (type 0) to describe isolated simple areola protrusion, either permanent or intermittent, that is associated with a normal mammary base (Table 3). The revised classification of tuberous breasts and the proposed hypothesis of breast development allow better assessment of all possible variants of breast morphological anomalies. In the cases of isolated herniated NAC, the deformity is corrected through a perinipple approach (not circumareolar), with adequate stable correction of the deformity and minimal scarring [173]. Many terms, including duct ectasia, secretory disease, periductal mastitis, and plasmacell mastitis, have been used about a variety of clinical conditions associated with nipple discharge, non-puerperal sepsis, and nipple retraction. The confused nomenclature reflects the uncertainties regarding the singularity or inter-relationships of the main elements – dilated ducts, periductal inflammation, bacterial infection, and nipple retraction. Recent clinical studies combined with new histological and bacteriological information have set the scene for better understanding of the pathogenesis and management of these clinical conditions [174]. Skin conditions affecting the nipple include eczema, which, while like eczema occurring elsewhere on the body, poses unique aspects in terms of diagnosis and treatment. The benign abnormalities that develop within the nipple include intraductal papilloma and nipple adenoma [175, 176]. Nipple adenoma can pose a diagnostic problem for both clinicians and pathologists. It can be confused clinically with nipple Paget’s disease and histologically with carcinoma. Syringomatous nipple adenoma is often misdiagnosed because clinical examination and mammographic findings of syringomatous nipple adenoma mimic carcinoma. Most nipple adenomas are entirely benign, although rare examples have shown malignant changes. Despite its benign behavior, syringomatous nipple adenoma usually shows infiltrative expansile proliferation into adjacent tissue and underlying breast tissue. Up until now, to our knowledge, there has been no reported case of regional or distant 40 Ciro Comparetto and Franco Borruto metastasis. Histologically and clinically, syringomatous nipple adenoma is often confused with tubular carcinoma as well as low-grade adenosquamous breast carcinoma. Special attention given to this tumor by pathologists and clinicians can avoid misdiagnosis and unnecessary treatment. Limited surgical excision is the appropriate treatment [177, 178]. Table 3. Tuberous breasts classification (adapted from mybreastaugmentation.info, 2017) Type I – Underdevelopment (hypoplasia) of the lower medial quadrant. Type II – Underdevelopment of the lower medial and the lower lateral quadrants, but with sufficient skin in the region below the areola. Type III – Underdevelopment of the lower medial and the lower lateral quadrants, but with a deficiency of skin in the region below the areola (this is like Type II but with less skin below the areola, and a tight inframammary fold). Type IV – Severe breast constriction, with a very minimal breast base. Papillary secretion is a common symptom in the breast clinic with many different underlying causes like intraductal papilloma, mammary duct ectasia, infected cysts and abscesses, breast cancer/ CIS, and rarely pituitary adenoma. The association with cancer/CIS is one of the two main concerns of the patient and must always be ruled out. The second item is that spontaneous and ongoing discharge causes embarrassment due to staining the clothes. Nipple discharge accounts for approximately 5% of visits to a breast specialist surgical practice and may be encountered as the chief complaint by many other types of physicians. The clear majority of breast cancers originate in the ductal system, which prompted interest in the evaluation of the intraductal approach to breast cancer. Three evolving minimally invasive techniques for the evaluation of high-risk patients include ductoscopy, nipple aspiration, and ductal lavage. These have emerged as innovative fields of study that may have clinical applications. Nipple aspiration and ductal lavage fluid may be assayed for cytology, genomic, gene expression, and proteomic studies. Several different translational approaches are being undertaken to investigate the local microenvironment associated with the development and progression of breast carcinoma. Nipple aspiration fluid and ductal lavage offer the opportunity to study the local microenvironment of the ductal system, which is where most breast cancers originate. These powerful approaches to biomarker analysis could be applied to the prevention and treatment of breast cancer [179]. Nipple discharge disorders is a field in which there has been both increasing awareness on the part of patients and advances in management. Today, secretion from nipples can be classified according to its color, Benign Breast Diseases 41 cellularity, and biology. To be significant, a discharge should be true, spontaneous, persistent, and non-lactational. Moreover, there are methods to differentiate patients who require surgical intervention from those who do not. Surgically significant nipple discharges are watery, serous (yellow), serosanguineous, and bloody. Cytology smears of discharge material have helped to classify the cellular material, providing information about normality, atypia, and malignancy and about papillary formation of the exfoliated cells. Tests such as Hemoccult (Beckman Coulter, Inc., Brea, CA, USA) help to discover occult blood in the secreted fluid. Modern immunological tests can be performed on cytology smears where occurrence of high levels of carcino-embryonic antigen (CEA) could indicate a latent malignancy. Galactography investigation is today the state-of-theart approach to investigate patients with nipple discharge disorders and this examination can demonstrate the size, location, and extent of an intraductal abnormality. Modern high-resolution ultrasound techniques are helpful in visualizing intraductal disorders and are becoming a good complementary approach if not an alternative to traditional radiology techniques. Recently, even MRI galactography has been shown to be of diagnostic value, but not as informative as regular galactography. The most sophisticated investigation method, which can also be used therapeutically, is fiber-ductoscopy of the concerned duct in a breast. This technique, although expensive and in its infancy, is a fascinating and promising approach for inspecting the intraductal lumina [180]. Traditional treatment is surgical excision of the involved ductal system from which the discharge emanates. Ductal excision has been the only reliable procedure in establishing a certain diagnosis and in controlling the bloody discharge. The early success reported with imaging-guided excision of papilloma and duct endoscopy promises a significant improvement in our diagnostic accuracy from minimally invasive emerging technology [181, 182]. Surgery (when undertaken) due to benign discharge should aim for radical excision of the underlying cause of the discharge, sometimes only a dilated single duct with or without a papilloma or excision of the central ducts as in case of mammary duct ectasia. Discharge should be consistent, spontaneous, and disturbing otherwise surgery should not be recommended in the absence of a suspicion of malignancy (negative triple test) [183]. The benefits of breast-feeding are well known, and the World Health Organization (WHO) recommends exclusive breast-feeding for the first six months of life and continuing breast-feeding to age two. However, many women stop breast-feeding due to lactational breast abscesses. A breast abscess is a localized accumulation of infected fluid in breast tissue. Abscesses are commonly treated with antibiotics, incision and drainage (I&D), or ultrasound-guided needle aspiration, but there is no consensus on the optimal treatment. There is insufficient evidence to determine whether needle aspiration is a more effective option to I&D for lactational breast abscesses, or whether an antibiotic should be routinely added to women undergoing I&D for lactational breast 42 Ciro Comparetto and Franco Borruto abscesses [184, 185]. Periductal mastitis/duct ectasia affects major breast ducts and is poorly understood. A variety of different terms has been used for this condition and these probably reflect different stages in one disease process. It appears to be responsible for 1-2% of all symptomatic breast conditions. Although the incidence is higher in postmortem studies, much of what is included as so-called “periductal mastitis” or “duct ectasia” in these studies is duct dilatation, which occurs as part of normal breast involution. Periductal mastitis appears to be the primary condition, with duct ectasia being the outcome. The cause of this periductal mastitis is uncertain, although bacteria, particularly anaerobic organisms, appear to play some role. Clinically, this condition can present with non-cyclical mastalgia, nipple discharge, nipple retraction, a subareolar breast mass with or without overlying breast inflammation, a periareolar abscess, or a mammillary fistula. Antibiotics against the organisms isolated from this condition are effective in resolving periareolar inflammation and are useful when combined with surgery in mammillary fistula [186]. Idiopathic granulomatous mastitis is a rare chronic inflammatory condition of the breast which predominantly occurs in premenopausal women shortly after their last childbirth, and which although benign can mimic carcinoma. Its etiology is unclear, however, the disease has been shown to be correlated with breast-feeding and the use of oral contraceptives. An autoimmune component has also been discussed. It presents with the clinical symptoms of galactorrhea, inflammation, breast mass, tumorous indurations, and ulcerations of the skin. In mammography and sonography, nodular opacities and hypoechoic nodules are found. Very often, clinical and radiological findings mimic breast cancer. The diagnosis is made by histopathology. Histological features in granulomatous mastitis include signs of a chronic granulomatous inflammation with noncaseating granulomas which are of a lobulocentric pattern, giant cells, leucocytes, epitheloid cells, and macrophages as well as abscesses. Therapy of granulomatous mastitis consists of complete surgical excision combined with oral steroid therapy, eventually in combination with anti-inflammatory drugs or colchicine. Use of MTX has also been successful. In case of formation of abscesses, antibiotic therapy should be applied before steroid therapy. Immune-suppressive therapy should be performed until complete remission, as rates of recurrence can be up to 50% [187]. Management of confirmed cases remains controversial with proponents of initial surgical or medical therapies – each has its associated problems which can be worse than the original symptoms of granulomatous mastitis. However, many patients require more than one modality of treatment to completely resolve granulomatous mastitis lesions and careful judgment is necessary to ensure optimal type and sequencing of treatments [188]. Tubercular mastitis is a rare clinical entity as mammary gland tissue, like spleen and skeletal muscle, resists the survival and multiplication of the tubercle bacillus. Breast tuberculosis can mimic carcinoma, whereas in young patients it can be mistaken for a Benign Breast Diseases 43 pyogenic breast abscess, thus labeled a “great masquerade” in recognition of its multifaceted presentation. Breast tuberculosis commonly affects women in the reproductive age group, between 21 and 30 years, and is rare in prepubescent females and elderly women. FNAC is very useful and it is a promising technique in expert hands. In tuberculosis-endemic countries, the finding of granuloma on FNAC warrants empirical treatment for tuberculosis even in the absence of positive acid-fast bacilli and without culture results [189]. Approximately 25% of all “discrete” breast lesions are fibroadenomas or breast cysts and they more commonly cause a breast lump than breast cancer. Despite their frequency, their natural history and relationship to subsequent breast cancer have not been clearly defined, although palpable breast cysts, but not fibroadenomas, are associated with some increased risk of breast cancer. The diagnosis of these two entities is now possible by FNA and excision of these lesions is only indicated in certain circumstances [190]. Fibroadenomas of the breast are common, accounting for 50% of all breast biopsies performed. Fibroadenomas are benign tumors which commonly present in late adolescence and are the most common breast masses in adolescent women, therefore it is important that health providers understand their assessment and management. They are classified according to their histology and size. Simple fibroadenomas are the most common type and usually present as smooth mobile masses up to 3 cm in diameter. Giant fibroadenomas are more uncommon but typically present in adolescence. Multiple, giant fibroadenomas are histological and clinical variants of “juvenile” or “giant” fibroadenomas. These tumors are rare and occur mainly in adolescent and young adult black females. The individual lesions are well encapsulated with a histological pattern primarily of the “juvenile” type, although cases of the “adult” type have been reported. Fibroadenomas associated with other soft-tissue masses should raise the possibility of an inherited syndrome. Assessment of breast masses in this age group generally involves clinical assessment through history and physical examination and, when imaging is needed, ultrasonography. As the incidence of primary breast malignancy is very low in this age group, CNB is not routinely recommended. Transformation from fibroadenoma to cancer is rare, regression or resolution is frequent, supporting conservative approaches to follow-up and management. Large or rapidly growing tumors, or those associated with suspicious features, warrant surgical excision. New minimally invasive excision techniques are being introduced which are associated with high initial success rates. A high incidence of recurrence is noted upon local excision, although this may decrease as the patient becomes older. Management options include local excision with reconstruction, reduction mammoplasty, and simple mastectomy with reconstruction. Whereas the clear majority of fibroadenomas in teenagers may be monitored with surveillance alone, new minimally invasive techniques may play an important role in the management of selected patients [191-193]. 44 Ciro Comparetto and Franco Borruto Breast fibromatosis is a rare benign tumor that should be included in the differential diagnosis for breast cancer. It is usually indistinguishable from malignancy on ultrasound, mammography, physical examination, and on gross evaluation. Distinction is easily made by histological findings. This benign tumor does not metastasize, but is locally aggressive and tends to recur postoperatively, which accounts for considerable morbidity [194, 195]. Most women have fibrocystic changes in their breasts. Patients with proliferative changes with atypia are at an increased risk of breast cancer. The management of mastalgia is the challenge most frequently encountered in patients with fibrocystic changes. Clinically, the types and variants of fibrocystic changes are sometimes difficult to distinguish. When the diagnosis of fibrocystic changes is unclear, a histological evaluation biopsy, open surgical biopsy, should be performed [196]. The prominence of breast fibrocystic change mandates knowledge of pathophysiology, diagnosis, and treatment on the part of all providing primary care to women [197]. Human breast cystic disease is a common premenopausal benign breast condition. Apocrine metaplasia of normal breast epithelium is the lesion that allows cysts to develop. Apocrine metaplasia is a very common finding in the female breast after the age of 25. It is so common that many people regard it as a normal component of the breast. This, however, is only really the case in apocrine sweat glands of the axilla and in the periareolar apocrine glands. The apocrine cell does, however, contribute to many different breast lesions, some of which are very taxing diagnostically: apocrine variants of both in-situ and invasive cancer are encountered [198]. Apocrine metaplasia and breast cysts occur frequently in association with other proliferative changes in breast epithelium, especially breast epithelial hyperplasia. Clinical follow-up studies of women with breast cystic disease indicate an increased risk of subsequent development of breast carcinoma. This risk is enhanced when multiple cysts occur. A positive family history of breast carcinoma adds to the increased risk that is associated with breast cystic disease. Biochemical analysis of breast cystic disease fluid shows a unique protein profile. Gross cystic disease fluid protein 15 (GCDFP-15) in breast cystic disease fluid is also found by immune-peroxidase staining to be present in approximately 50% of all breast carcinomas. Enhanced production of GCDFP-15 by breast carcinomas has been shown experimentally and clinically with the use of androgens [199]. Most evidence on management of breast cysts was obtained from cohort studies. Family physicians can manage women presenting with breast lumps if they have skill in breast cyst aspiration. Most breast cysts can be cured in minutes, thus avoiding unwarranted anxiety and eliminating unnecessary additional investigations and referrals. Women presenting with solid lesions should be referred to a surgeon. Breast cyst aspiration is a simple technique that family physicians can use to either cure breast lumps or define appropriate cases for referral [200]. Benign Breast Diseases 45 Papillary breast lesions encompass a wide spectrum of pathologies ranging from benign lesions, such as solitary intraductal papilloma, to the uncommon papillary carcinoma, whose hallmark is the formation of either papillary tufts composed only of epithelium, or true papillary structures with fibrovascular support and epithelium. The precise diagnosis has significance because of the variability in treatment modalities. These lesions have various clinical presentations and diverse radiological features. Differentiating benign and malignant papillary lesions based on imaging features may often be difficult. Other benign and malignant pathologies can also mimic papillary lesions on imaging, and tissue diagnosis is essential. Imaging plays a key role in lesion identification, assessment of extent, tissue sampling, and follow-up. Surgical excision has been recommended for all papillary lesions due to an increased incidence of highrisk lesions and neoplasia even with percutaneous, biopsy-proven benign papillomas [201, 202]. The condition of multiple breast duct papillomas, although relatively common in adult women, is not a well recognized clinical entity in adolescent females. The risk of adolescents with this condition developing carcinoma is presently unknown, although in adulthood it is regarded by some authorities as a precancerous condition [203]. The assessment and categorization of papillary lesions remains one of the most challenging areas in breast pathology. Several diagnostic and management issues related to papillary breast lesions that are frequently encountered in daily practice include: 1) the distinctions among papillomas with atypia (atypical papillomas), papillomas with DCIS, and papillary DCIS; 2) recent developments in our understanding of encapsulated (“intracystic”) papillary carcinomas and solid papillary carcinomas; and 3) the impact of CNB on management decisions and specimen evaluation [204]. Obtaining a balance between overtreatment and undertreatment of these lesions is also challenging. Knowledge of the clinical presentation, histology, immune-profile, and behavior of papillary breast lesions will assist pathologists with the diagnosis and optimal management of patients with papillary breast lesions [205]. Interpretation of breast papillary lesions remains a challenging task because of the wide morphologic spectrum encountered in the benign, atypical, and malignant subtypes. Data on clinical significance and outcome of papillary lesions, with superimposed atypia or areas like DCIS partially replacing the benign elements, are sparse. Furthermore, complete excision of even a fully developed papillary carcinoma confined to a dilated or cystic duct is associated with an excellent prognosis, whereas a complex papilloma extending into multiple branches of a duct may ultimately recur as a carcinoma because of incomplete excision of microscopic foci. This makes an outcome-based classification difficult. It would be prudent to completely excise any papillary lesion that has not been entirely 46 Ciro Comparetto and Franco Borruto removed by the initial CNB. The optimal management of localized papillary lesions is complete excision with a small rim of uninvolved breast tissue without any prior needle instrumentation when the papillary nature can be determined by imaging. Thus managed, most of these lesions behave indolently, and outcome is usually excellent [206]. The pathologist evaluating breast biopsy specimens sometimes encounters nonneoplastic alterations of the mammary epithelium that raise the differential diagnosis of atypia. Because the identification of breast atypical ductal hyperplasia has significant clinical implications, it is important to correctly recognize its non-neoplastic morphological mimics. Among physiological alterations of the ductal epithelium, lutealphase changes and secretory changes can sometimes be overinterpreted as atypical. Treatment-related changes, secondary to chemotherapy and radiation, can pose a major diagnostic challenge and their misinterpretation as neoplastic carries major clinical consequences. Familiarity with the morphology of both physiological and treatmentrelated alterations of the mammary epithelium is essential to avoid misdiagnosis [207]. High-risk benign breast lesions can create confusion for both the patient and the clinician and offer varying degrees of increased risk for the future development of breast cancer. Chemoprevention may be used to help decrease the risks from some lesions. The management of high-risk benign breast lesions can be confusing. Clinicians should assess the risk of future breast cancer and develop a proper screening and prevention strategy for each individual patient [208]. During the last several years, increased public awareness, advances in breast imaging, and enhanced screening programs have led to early breast cancer detection and attention to cancer prevention. The number of imaging-detected biopsies has increased, and pathologists are expected to provide more information with smaller tissue samples. These biopsies have resulted in detection of increasing numbers of high-risk proliferative breast disease and in-situ cancers. Atypical epithelial hyperplasia, LCIS (lobular neoplasia), radial scar, and DCIS are considered high-risk lesions that predispose toward the future development of non-invasive or invasive breast cancer. The general hypothesis is that some forms of breast cancers may arise from established forms of DCIS and atypical ductal hyperplasia, and possibly from more common forms of ductal hyperplasia. However, this is an oversimplification of a very complex process given the fact that most breast cancers appear to arise de novo or from a yet unknown precursor lesion. Currently, atypical ductal hyperplasia and DCIS are considered as morphological risk factors and precursor lesions for breast cancer. However, morphological distinction between these two entities has remained a real issue that continues to lead to overdiagnosis and overtreatment. Aside from morphological similarities between atypical ductal hyperplasia and low-grade DCIS, biomarker studies and molecular genetic testing have shown that morphological overlaps are reflected at the molecular level and raise questions about the validity of separating these two entities. It is hoped Benign Breast Diseases 47 that as we better understand the genetic basis of these entities in relation to ultimate patient outcome, the suggested use of the term “borderline breast disease” can minimize the number of patients who are subject to overtreatment [209]. Generally, those women with atypical epithelial hyperplasia, radial scar, or LCIS can be managed conservatively by close surveillance. The minority of women may consider prophylactic mastectomy. DCIS can usually be managed by lumpectomy with or without radiation, with some patients requiring mastectomy due to extensive disease [210]. This progress, however, has created a challenge for pathologists. In lieu of the fact that these entities are difficult to diagnose even in tissue sections taken from surgically excised lesions, pathologists are now expected to diagnose them in small and often fragmented tissue/cellular samples obtained from imaging-guided biopsies. In addition, some proliferative lesions are associated with an increased risk of finding neighboring malignancy when diagnosed on minimally invasive procedures. Therefore, classifying these lesions in small biopsies is difficult and risky. Some of the most challenging areas in diagnostic pathology includes the differentiation between atypical ductal hyperplasia and low-grade DCIS, lobular neoplasia versus solid low-grade DCIS, the correct interpretation of papillary lesions with atypia, and classifying the spectrum of columnar cell changes. Although these issues have been recognized for years, consensus criteria and uniform terminology for the diagnosis of these problematic lesions are far from being achieved [211]. Intraductal proliferative lesions (intraductal hyperplasia, atypical intraductal hyperplasia, and DCIS) should be classified as “mammary intraepithelial neoplasia (MIN), ductal type” or as “ductal intraepithelial neoplasia” (DIN). This approach obviates the current separation of atypical intraductal hyperplasia and low-grade DCIS into two very drastically distinct categories of cancer and non-cancer without interfering with appropriate management of the various lesions [212]. Breast atypical epithelial hyperplasia is considered a benign histological lesion with breast cancer risk. Clinical signification and management of atypical epithelial hyperplasia remain controversial. Data from immune-histochemistry (IHC) and molecular studies suggest atypical epithelial hyperplasia is a precursor of breast cancer. Epidemiological studies demonstrate low rate of breast cancer in women with atypical epithelial hyperplasia. Surgical excision is necessary after diagnosis of atypical epithelial hyperplasia, such as LCIS or atypical ductal hyperplasia, on CNB. Although current recommendations are evolving to fewer (if not no) excisions for flat epithelial with atypia and classic lobular neoplasia found on percutaneous biopsy (without radiological indications for excision), atypical epithelial hyperplasia management steel need prospective evidences, but recent retrospective data give some clue for less invasive management for some of atypical epithelial hyperplasia [213]. “Flat epithelial atypia” is the adopted term by the WHO working group on breast tumor referring to an early neoplastic breast lesion affecting the terminal duct-lobular units. Pathologists have described this lesion under a variety of 48 Ciro Comparetto and Franco Borruto names, including columnar cell lesions and low-grade clinging CIS. It is usually encountered on breast biopsies performed for mammographically-identified microcalcifications. Because of its relatively frequent association with carcinomas, its recognition in biopsy specimens is important [214]. Flat epithelial atypia is a presumably neoplastic alteration that is characterized by the replacement of the native luminal epithelium by ductal cells demonstrating low-grade cytologic atypia. The atypical cells maintain a “flat” pattern of growth without evidence of architectural atypicality. Morphologic IHC and molecular investigations support that flat epithelial atypia represents an early step in the evolution of low-grade ductal carcinomas. It is frequently seen in association with atypical ductal hyperplasia, low-grade DCIS, invasive tubular carcinoma, and lobular neoplasia. The risk for subsequent breast carcinoma remains to be defined, but flat epithelial atypia likely represents a non-obligate precursor with an extended time course to progression. Certain benign alterations may superficially mimic its appearance: careful attention to cytological and architectural characteristics can help one distinguish these unrelated entities from flat epithelial atypia [215, 216]. Breast columnar cell lesions refer to the morphological spectrum of alterations of the epithelial lining of variably dilated acini of the terminal duct lobular unit (TDLU), lined by one to several layers of tightly packed, columnar-shaped epithelial cells, often related to secretions and calcifications. After decades of varied terminologies, the term of “flat epithelial atypia” by the WHO consensus group encompasses the part of the spectrum where columnar cell change or columnar cell hyperplasia acquires low-grade cytological atypia, merging with atypical ductal hyperplasia and low-grade DCIS. Its association with low-grade invasive carcinoma and lobular neoplasia, whether by proximity to these lesions, or by similar molecular expressions, has prompted greater scrutiny into its clinical significance. Although recent literature attempts to refine the term “flat epithelial atypia,” the applicability of its morphological criteria in routine diagnostic practice remains to be seen, and interobserver variability is highly possible. This poses even greater challenges especially in limited samples of breast tissue, such as in CNB, for preoperative decision-making [217-219]. Lobular neoplasia broadly defines the spectrum of changes within the lobule, ranging from atypical lobular hyperplasia to LCIS. This continuum of lesions is associated with an increased risk for developing subsequent invasive breast cancer, with the magnitude of that risk corresponding to the degree of proliferative change. The associated risk for developing invasive breast cancer after a diagnosis of lobular neoplasia is multicentric, bilateral, and equal in both breasts. Lobular neoplasia itself may transform into invasive carcinoma, although the frequency of this occurrence is unknown. Thus, lobular neoplasia is a risk factor for invasive breast cancer and may be a precursor lesion in unusual circumstances. Although classic forms of lobular neoplasia are predominantly heralded as a risk marker, the pleomorphic form of LCIS is generally regarded as a more Benign Breast Diseases 49 aggressive subtype and a possible cancer precursor, and thus is treated in a manner more like DCS than classic forms of lobular neoplasia. Correct classification of classic lobular neoplasia and pleomorphic LCIS is critical because of differences in clinical management, with current treatment strategies focused on risk reduction for patients with classic lobular neoplasia and eradication of the lesion for those with pleomorphic LCIS [220]. The management of atypical lobular hyperplasia and LCIS depends on the setting in which they are encountered. When atypical lobular hyperplasia and LCIS are diagnosed after breast CNB, wire localization for surgical excision is required for definitive diagnosis because rates of histological underestimation approach those of atypical ductal hyperplasia. When diagnosed on surgical biopsy, atypical lobular hyperplasia and LCIS generally do not require further intervention, even when present at a surgical margin. However, bilateral breast cancer risk must be considered, especially when patients have a family history of breast cancer. In selected situations, bilateral prophylactic mastectomy with or without reconstruction may be considered when atypical hyperplasia or LCIS is diagnosed. Although this reduces risk for developing subsequent breast carcinoma by 90%, patients selected for prophylactic mastectomy represent a small subgroup of lobular neoplasia patients and generally have other risk factors, such as strong family history or evidence of genetic predisposition [221]. Lobular neoplasia is a relatively uncommon lesion, which is frequently diagnosed in biopsy specimens taken for other reasons. Although the histological features of this lesion are well known, its biological significance as a “risk indicator” or “breast cancer precursor” has been a matter of debate. Recent clinical-pathological and genetic molecular data on lobular neoplasia have changed the way these lesions are perceived and, most importantly, managed [222-224]. However, this opportunity comes with the possibility of overdiagnosis and overtreatment, necessitating risk-based intervention. Notably, despite the progress in defining the molecular changes associated with carcinogenesis, alterations identifying the individuals with high-risk lesions that will progress to invasive carcinoma remain to be identified. Thus, until reproducible clinicalpathological or molecular features predicting an individual’s risk of breast cancer are found, management strategies must be defined by population-level risks as determined by models such as the Gail or the International Breast Cancer Intervention Study (IBIS) models, as well as patient attitudes toward the risks and benefits of interventions. Progress in this area will ultimately be dependent on the ability to individualize risk prediction through better definition of the key drivers in the carcinogenic process [225]. Histopathologists are encountering intralobular epithelial proliferations more frequently in CNB taken from lesions identified in mammographic breast screening programs. Columnar cell lesions are increasingly being seen in CNB taken for the histological assessment of mammographically detected microcalcifications. Of the biopsies performed because of microcalcifications on mammograms, 70-90% are unnecessary. 50 Ciro Comparetto and Franco Borruto Most of the time descriptions of diagnostic criteria are irrelevant and vague. It is confusing that LCIS is considered a real cancer, as happens frequently, and that histologically dissimilar ductal carcinomas and benign abnormalities of different origin are simply considered as “malignant” or “benign” and subsequently reported as such. The number of needless biopsies can be reduced drastically [226]. The morphological features of lobular neoplasia are relatively well known, but columnar cell lesions, particularly forms with atypical features, are less widely recognized. Lobular intraepithelial neoplasia (LIN) and DIN reflect proliferations of immune-phenotypically variable, biologically and morphologically diverse cells with a potential, not always realized, for progression to carcinoma by breaking through the barriers of the myoepithelial cell layer and basement membrane, ultimately invading the stroma [227]. The biological and clinical significance of these intralobular processes is controversial: 1) as indicators of adjacent malignancy when encountered in CNB; 2) the relative risk (RR) conferred of development of subsequent malignancy; and 3) their precursor behavior. For this reason, the optimal clinical management of these lesions, particularly when encountered on CNB, is unclear [228, 229]. Radial scar is a benign, well recognized, radiological and pathological entity. Histologically, it is characterized by a fibroelastotic core with entrapped ducts and surrounding radiating ducts and lobules. Post-mortem studies indicate that these lesions are present commonly in the population, especially in association with benign breast disease. In recent years, their clinical relevance has assumed more importance with the introduction of population-based screening programs. The exact pathogenesis of radial scar is unknown. Accumulating evidence indicates that they are associated with atypia and/or malignancy and, in addition, may be an independent risk factor for the development of carcinoma in either breast. In view of the association with atypia and malignancy, excisional biopsy is justified in radial scar, although it has been argued that CNB evaluation and surveillance may be appropriate in selected patients [230]. The management of precancerous lesions of the breast has become a considerable clinical problem in the past 20 years. It is not possible to identify with absolute certainty which of these lesions will progress to invasive carcinoma, and tailoring the treatment according to each individual case remains a challenge. There is a dilemma for surgeons, who must balance the risk of resecting too much and causing unnecessary cosmetic damage, or resecting too little and leaving an increased risk of recurrence. Further knowledge in the field of predictive and prognostic factors together with the development of gene-profiling techniques will, hopefully, provide answers to these questions. Nearly all possible combinations of surgery, radiotherapy, and medical Benign Breast Diseases 51 treatments (antiestrogens) have been tested in different clinical trials, but the situation is far from satisfactory. An important contribution can come from oncoplastic surgery, which is the application of plastic and reconstructive surgical techniques to ensure both radical excision of the disease and acceptable cosmetic outcomes. Although the relationship of various breast lesions and cancer risk is complex, and is compounded by the uniqueness of each patient, numerous clinical investigations have validated the implications of specific lesions and future risk. While variations in lesion cytology, histology, and molecular biology present a challenge to the clinician in determining disease risk and formulating a management strategy for individual patients, research has yielded several guidelines. These include patient variables, such as age and menopausal status, as well as information concerning the lesion itself [231-235]. Pseudoangiomatous stromal breast hyperplasia is a benign, proliferative mesenchymal lesion with possible hormonal etiology. It typically affects women in the reproductive age group. The exact etiology of pseudoangiomatous stromal hyperplasia is still controversial, but a neoplastic process of the stromal myofibroblasts, with a hormonal stimulus in its development and progression, is the favored theory. Pseudoangiomatous stromal hyperplasia is frequently an incidental histological finding in breast biopsies performed for other benign or malignant lesions. Rarely, it can present as a firm, painless breast mass, which has been referred to as nodular or tumorous pseudoangiomatous stromal hyperplasia. Grossly, tumorous pseudoangiomatous stromal hyperplasia is a well-circumscribed, firm, and rubbery mass with solid, homogenous, and gray-white cut surface. On histological examination, it is characterized by the presence of open slit-like spaces in dense collagenous stroma. The spaces are lined by a discontinuous layer of flat, spindle-shaped myofibroblasts with bland nuclei. The spindle cells express PR and are positive for vimentin, actin, and cluster D 34 (CD34). The clinical, radiological, and cytological findings can resemble those of fibroadenoma. The most important differential diagnosis on histopathology is angiosarcoma. Pseudoangiomatous stromal hyperplasia discovered incidentally does not require any additional specific treatment. Tumorous pseudoangiomatous stromal hyperplasia is treated by local surgical excision with clear margins and the prognosis is excellent, with minimal risk of recurrence after adequate surgical excision [236, 237]. Phyllodes tumors is a rare fibroepithelial lesion that accounts for less than 1% of all breast neoplasms with a very variable, but usually benign, course. Formerly known as cystosarcoma phyllodes, the designation “phyllodes tumor” with appropriate qualification regarding malignant potential based on pathological features is now the agreed-upon term. The most important diagnostic distinction is from fibroadenoma – phyllodes tumors require complete excision with free margins even when pathological features suggest benignity because of a proclivity to local recurrence. As CNB cannot always distinguish the two, assessing radiological-pathological concordance is essential 52 Ciro Comparetto and Franco Borruto to guide appropriate clinical management. With the non-operative management of fibroadenomas widely adopted, the importance of phyllodes tumors today lies in the need to differentiate them from other benign breast lesions. All breast lumps should be tripleassessed and the diagnosis of a phyllodes tumor considered in women, particularly over the age of 35 years, who present with a rapidly growing “benign” breast lump. The main prognostic factor is the histotype of the phyllodes tumor (benign, borderline, or malignant). The role of the pathologist in the preoperative diagnosis of breast phyllodes tumors is critical to appropriate surgical planning. However, reliable differentiation of phyllodes tumor from cellular fibroadenoma remains difficult. Preoperative diagnostic accuracy allows correct surgical treatment, avoiding the pitfalls of re-operation because of inadequate excision, or surgical overtreatment. Specific clinical indices may arouse diagnostic suspicion but are unreliable for confirmation, as with current imaging modes. FNAC has a high false-negative rate. Few studies have evaluated the role of CNB, but it may prove a useful adjunct. Both diagnostic and prognostic information may in future be gained from application of IHC and other techniques assessing the expression of proliferative markers including p53, Ki-67, and others. Treatment can be by either wide excision or mastectomy provided histologically clear specimen margins are ensured. While the surgical management of these relatively uncommon tumors has been addressed in the literature, few reports have commented on the surgical approach to tumors greater than 10 cm in diameter – the giant phyllodes tumor. Management of the giant phyllodes tumor presents the surgeon with unique challenges. Nodal metastases are rare and routine ALND is not recommended. Few reliable clinical and histological prognostic factors have been identified. Local recurrence occurs in approximately 15% of patients and is more common after incomplete excision. It can usually be controlled by further surgery. Repeated local recurrence has been reported without the development of distant metastases or reduced survival. Approximately 20% of patients with malignant phyllodes tumors develop distant metastases. Long-term survival with distant metastases is rare. The role of chemotherapy, radiotherapy, and hormonal manipulation in both the adjuvant and palliative settings remains to be defined. At present, there is no consensus that patients with high-grade phyllodes tumors of the breast will benefit from either of these modalities [238-243]. Breast myofibroblastoma is an unusual benign tumor that belongs to the family of the “benign spindle cell tumors of the mammary stroma.” The name myofibroblastoma reflects its cellular composition, comprising mainly stromal cells with myofibroblastic and, less frequently, myoid differentiation. Since the original description, the morphologic spectrum of myofibroblastoma has been expanded by the recognition of several unusual morphological variants, such as the cellular, infiltrative, epithelioid, deciduoid-like, lipomatous, collagenized/fibrous, and myxoid variants. Myofibroblastoma may show alarming morphological features, which can lead to a Benign Breast Diseases 53 misdiagnosis of malignancy. The incidence of myofibroblastoma diagnosis has increased in recent years, likely due to the mammographic screening. Accordingly, this unusual benign tumor may represent a potential diagnostic pitfall, especially when interpreting FNA and/or CNB. Pathologists should be aware of the wide morphological spectrum exhibited by myofibroblastoma to avoid a misdiagnosis of malignancy [244]. Fat breast necrosis is a benign condition that most frequently affects perimenopausal women. It can mimic breast cancer clinically or radiologically. In other cases, it can obscure malignant lesions. Fat breast necrosis is a complex process. Therefore, a systematic review of this condition will enable surgeons, radiologists, and oncologists working in the field of breast disease to understand it better and improve its management [245]. Hibernoma arising in the breast is rare and may present as an asymptomatic mass or may be detected by screening mammography. Four histological types have been identified: typical, myxoid variant, spindle-cell variant, and the lipoma-like variant. The most common “typical variant” is composed of pale to eosinophilic multivacuolated cells with interspersed univacuolar cells. Hibernomas are universally benign and are not known to recur or have an aggressive behavior, even in incompletely excised lesions. Hence, their clinical importance lies in distinguishing them from other benign and malignant breast neoplasms as well as inflammatory conditions that come into the histological or radiological differential [246]. Sclerosing lymphocytic lobulitis is strongly associated with long standing type 1 diabetes, then it is known as “diabetic mastopathy,” but may occur occasionally in its absence. This very rare benign condition tends to present in premenopausal women, often with diabetic complications, particularly retinopathy and neuropathy, but more research is needed to verify this association. Patients present with clinically suspicious fibrous breast lumps: these are commonly multiple, bilateral, and recurrent, usually in a subareolar site, but may appear equally in any part of the breast. Mammograms show asymmetric densities and no focal mass and ultrasound investigation tends to show illdefined hypoechoic attenuation with strong posterior acoustic shadowing. Mammographically and morphologically, this lesion simulates cancer. MRI can be used to further differentiate the lesion from malignancy. However, a CNB or excisional biopsy is essential so that a pathological diagnosis can be made. The constellation of pathological findings is: lymphocytic lobulitis and ductitis with glandular atrophy, lymphocytic/mononuclear perivascular inflammation which is predominantly B-cell, and dense often keloid-like fibrosis, with or without epithelioid-like fibroblasts. The etiopathogenesis is unknown and the disorder does not seem to predispose to breast carcinoma or lymphoma. The lesion may recur after excision in the same site or in another location of the ipsilateral or the contralateral breast. Awareness of this entity, establishment of the diagnosis by open biopsy or by CNB may spare the need for repeated wide excisions and the resulting distortion of the breast architecture [247-249]. 54 Ciro Comparetto and Franco Borruto In conclusion, the purpose of breast screening is to identify early cancers with the aim of reducing mortality from the disease. However, the specificity of mammography is such that a considerable number of women will require assessment for benign disease, particularly in the prevalent round. Knowledge of the types of benign breast disease which are commonly seen in screening, together with careful assessment using the techniques which have been described, will allow the benign to malignant biopsy rates to be kept acceptable without missing the very lesions which screening hopes to identify [250]. Chapter IV Breast Cancer Breast cancer, while often lumped together as one disease, represents a diverse group of malignancies with different imaging findings, histological appearances, and behavior. While most invasive primary breast cancers are epithelial derived adenocarcinomas, rare neoplasms may arise from mesenchymal tissue. Breast cancer has become the top malignant neoplasm in women with an increasing risk of morbidity and mortality. As a crucial part of comprehensive treatment of breast cancer, breast surgical technique is ceaselessly ameliorating and enriching its features. With the purpose of achieving minimal surgical intervention and satisfactory cosmetic results, the trend of mammary surgery is focusing on minimally invasive treatment and aesthetics in the 21st century [251-253]. Personalized medicine is emerging as an important guiding principle in diagnosis and treatment. This means not just doing more for some, but safely doing less for others. The lessons learned about the biology of breast cancer over the last two decades have enabled us to understand the incredible heterogeneity of breast cancer and its associated behavior. Although much work remains, there is an emerging opportunity to identify and recognize more indolent forms of breast cancer, made more prevalent through the widespread adoption of screening. With improving systemic therapies and improved molecular tools, we now have the opportunity to reduce the burden of treatment in women with lower-risk tumors. Surgical treatments have evolved, with less morbid and more cosmetic procedures [254]. Although controversy has emerged in recent years regarding the diagnosis and treatment of this disease, it remains important to detect and treat breast cancer before it has metastasized [255, 256]. The surgical management of breast cancer has undergone continuous and profound changes over the last 40 years. The evolution from aggressive and mutilating treatment to conservative approach has been long, but constant, despite the controversies that appeared every time a new procedure came to light. Today, the aesthetic satisfaction of 56 Ciro Comparetto and Franco Borruto breast cancer patients coupled with the oncological safety is the goal of the modern breast surgeon. Breast-conserving surgery with adjuvant radiotherapy is considered the gold standard approach for patients with early-stage breast cancer and the recent introduction of “oncoplastic techniques” has furtherly increased the use of breastconserving procedures. Mastectomy remains a valid surgical alternative in selected cases and is usually associated with immediate reconstructive procedures. New surgical procedures called “conservative mastectomies” are emerging as techniques that combine oncological safety and cosmesis by entirely removing the breast parenchyma sparing the breast skin and NAC. Staging of the axilla has also gradually evolved toward less aggressive approaches with the adoption of SLNB and new therapeutic strategies are emerging in patients with a pathological positivity in SLNB [257]. Local treatment of breast cancer with tumor-free surgical margins is the standard procedure in the treatment of T1 and small T2 breast cancers. Surgery is followed by radiation therapy, and adjuvant systemic therapy is offered depending on primary tumor characteristics, such as tumor size, grade of differentiation, number of involved ALN, the status of ER and PR, and the expression of the HER-2 receptor. Although this approach implies a higher risk of ipsilateral breast tumor recurrence, the total risk of recurrence is low (1% per year), with rates of overall survival similar to that after radical procedures. The most peripheral part of epithelial tumors, the tumor margin, is the part which is most likely to remain in loco after surgical resection. Thus, understanding the biology of the invasion front is important as these tumor cells have been reported to lose epithelial properties, such as cohesiveness and keratin expression, and to acquire features of mesenchymal cells. The parallel appearance of tumor cells in different states of cell dedifferentiation implicates a dynamic equilibrium that is determined by the induction of epithelial-mesenchymal transition (EMT). EMT has been suggested to be of prime importance for tissue and vessel invasion. Furthermore, features of EMT are associated with the activity of tumor stem cells (TSC). TSC exist in breast cancer and their appearance varies depending on the used marker profile. Consequently, intratumoral heterogeneity is reflected by the grade of EMT activation. A specific function at the invasion front is hypothesized but has not yet been proven. Nevertheless, the molecular differentiation between the tumor center and the invasion front enhances the importance of tumor-free surgical margins [258-261]. The recent history of operations for breast cancer indicates a growing trend toward conservatism. The modified radical mastectomy achieves the goal of removing all evidence of cancer in the breast involved and removes the regional lymph nodes (RLN) for accurate staging of the disease. In addition, it provides a cosmetic result superior to that of the standard radical mastectomy. Breast reconstruction may be undertaken later with excellent result [262]. The surgical management of breast cancer continues to evolve in an attempt to define the ideal line between therapeutic efficacy and morbidity. Breast Cancer 57 It is clear that breast cancer is a biologically heterogeneous group of diseases, and no single hypothesis explains its behavior. The surgical options proposed to the individual patient must draw from the experience of retrospective clinical studies and prospective RCT in an attempt to optimize the treatment plan. Most patients without distant disease are eligible to consider mastectomy, which can accomplish excellent local control and significantly improve survival for earlier stages of disease. However, breast conservation remains an appropriate alternative for a carefully defined subset of patients. Today, with early-stage disease, no patient need leave the operating room without a breast. Recent advances in reconstructive surgery make mastectomy with immediate reconstruction or limited resection plus ALND with postoperative radiation therapy the two principal treatment choices available. Studies focus on the integration of other treatment modalities. Clinical research into the use of preoperative chemotherapy to downstage the disease to permit less extensive surgery is of interest. Recent application of molecular biological techniques such as oncogene analysis, cytogenetic studies, proliferative indices, and the highly sensitive detection of distant micrometastases using monoclonal antibodies may assist in the design of innovative approaches to surgery, radiation therapy, and systemic drug treatment. These advances show great promise for improving the quality of life and the cure rate for patients with breast cancer. Today, surgical treatment options have evolved that fulfill some of the objectives outlined by Dr. James Ewing of Memorial Hospital some 80 years ago. His concerns about breast cancer remain as relevant today as they were almost a century ago: “I have drawn the impression that in dealing with mammary cancer, surgery meets with more peculiar difficulties and uncertainties than with almost any other form of the disease. The anatomical types are so numerous, the variations in clinical course so wide, the paths of dissemination so free and diverse, the difficulties of determining the actual conditions so complex, and the sacrifice of tissues so great, as to render impossible in the majority of cases a reasonably accurate adjustment of a means to an end” [263]. A very satisfying concept of treatment is not easy to formulate from the complex and often conflicting results of local therapeutic interventions for breast cancer. It seems evident that clinically occult cancer is often beyond the pale of both resection and irradiation at primary treatment, particularly when cancer is found in RLN. Despite all combinations of local treatment, the ultimate risk of failure correlates more closely with the stage of the disease at the time of treatment than with the particular form of treatment. Thus, the extent of disease must be considered the major, perhaps the ultimate determinant of prognosis. Since, under controlled conditions, several therapeutic alternatives have appeared to provide virtually identical results in terms of survival and ultimate dissemination of the disease, the adequacy of control within the field of treatment, in fact, may be the most meaningful result of local treatment. The experience that has accumulated with treatment of breast cancer supports the thesis that removal of 58 Ciro Comparetto and Franco Borruto the breast accomplishes all that can be achieved in terms of curing the disease, and wider treatment with surgery or irradiation serves only to improve the prospects for local control. Halsted demonstrated this principle with his radical mastectomy and it still seems to be the case. This fact provides further impetus for detecting and treating cancer while it is still localized to the breast. With these generalizations in mind, some empirical observations can be added. An anatomical fact is that multiple microscopic foci of cancer that are not evident clinically are often present in the mammary parenchyma. Undisturbed, at least some, and perhaps eventually all, of these foci of cancer progress to become clinical cancers. Thorough removal of the entire breast (the entire mammary parenchyma) eliminates this particular hazard and, one may presume, terminates the disease if it is still limited to the breast. Removal of the underlying pectoralis major muscle provides additional margin around the tissues primarily involved, but sacrifice of the muscle is apparently needless unless it is directly invaded by cancer. Microscopic metastases are also often present in RLN without being clinically detectable and, left untreated, have the capacity to enlarge and become clinically apparent. Routine wide removal of RLN improves the control of cancer at these sites when metastases are present, but whether it improves the chances for cure is doubtful. The fact is that approximately 25% of patients with ALN metastases enjoy prolonged survival free of recurrence, some remaining well even after 30 years. Whether they would survive as well without removal of the metastases is uncertain. Disease-free survival is highest if metastases are removed while still microscopic, but this phenomenon may simply reflect treatment at an earlier phase in the evolution of the disease [264, 265]. The clinical assessment of patients with Stage I and II breast cancer is limited to a history and physical examination supplemented by a breast radiograph and serum alkaline phosphatase (ALP) determination. If the serum ALP is abnormal in the presence of otherwise normal liver function studies, a bone scan, liver scan, and CEA should be obtained. Areas of increased radioactivity on bone scan are always evaluated by additional radiographs and tomograms. The majority of focal areas of increased radioactivity will demonstrate radiographic evidence of benign bone lesions, predominantly degenerative joint disease. Only those focal areas of increased radioactivity that are normal on X-ray film or show radiographic evidence of metastases are considered to be positive for metastatic disease. The results of the liver scan are correlated with the level of CEA. Focal areas of decreased radioactivity associated with a CEA greater than 5 nanograms/milliliter (ng/ml) are considered to be metastases. In the absence of elevation of the CEA, focal areas of increased radioactivity should be biopsied prior to any further considerations as to definitive therapy. In the absence of positive clinical findings or elevation of the ALP, routine scintigraphy of the skeletal system and liver is not indicated. Patients with Stage III disease have a much greater chance of having clinically occult metastases of sufficient size to be detected by chest X- Breast Cancer 59 ray film, serum ALP, and bone scan. In contrast, routine scintigraphy of the skeletal system and liver is indicated in patients with Stage III disease, even in the absence of clinical evidence of systemic metastasis. If the serum ALP is abnormal, a liver scan and CEA are obtained in an effort to detect liver metastases. The same sequence of events is then followed as suggested for patients with Stages I and II disease. Several new techniques of detecting occult metastases are being evaluated. Biomarkers are the subject of several studies. The use of computed tomography (CT) is also being utilized as a means of detecting lung, liver, and mediastinal metastases. The results of clinical trials should be carefully followed [266, 267]. CIS of the breast is divided into DCIS and LCIS. These two entities have different morphological appearances and natural histories. The traditional treatment of DCIS has been mastectomy, which has been shown to yield a very high cure rate. Other options include excision with or without radiotherapy. Preliminary evidence suggests that these techniques can be successful in the great majority of patients, with most treatment failures being manageable by salvage mastectomy. Careful observation of these patients appears associated with a low risk of breast cancer mortality and is generally the recommended policy [268]. Both DCIS and LCIS are forerunners of invasive breast cancer but with different behavior. Neither condition is fatal but management can be difficult. After 20 years follow-up of LCIS, the cumulative risk of ipsilateral invasive cancer is 18%, and 14% for contralateral disease. Of the cancers that occur, 40% are invasive lobular and 60% invasive ductal. Standard management of LCIS is surveillance, with no attempt to perform a wide excision or a mastectomy. Many cases of DCIS are detected because of microcalcification on mammograms: 20% of screen-detected cancers are of this type. DCIS is a forerunner of ipsilateral invasive disease, so complete excision is necessary. Subsequent relapse or progression to invasive disease can be reduced by breast irradiation. Because of the extent of DCIS, a significant proportion of patients will need a total mastectomy with or without immediate reconstruction [269-271]. The clinical expression of in-situ cancer varies widely but is usually occult. Diagnosis can be made by a variety of minimally invasive techniques. Treatment of LCIS is patientdirected but generally requires only close follow-up. Mastectomy is the gold standard for DCIS and is associated with low recurrence rates. Breast conservation therapy has become an acceptable alternative. This choice of definitive therapy for DCIS depends largely on the ability to obtain negative margins. Any attempt at breast conservation therapy should be coupled with the caveat of close postoperative long-term follow-up. Patients diagnosed with LCIS or who have a history of DCIS should be given the options for the use of TMX for the reduction of subsequent development of invasive breast cancer. Risk versus benefits should be clearly defined [272]. DCIS is an early localized stage of mammary malignancy that has an especially favorable prognosis with appropriate management. It seems to be a direct precursor to 60 Ciro Comparetto and Franco Borruto invasive carcinoma of the breast and has the same therapeutic options. There are still many outstanding issues to be resolved before the intriguing potential of this disease can be fully realized [273]. DCIS is increasing in frequency, primarily because of the increasing use of routine screening mammography. The management of DCIS has become one of the more controversial aspects in the treatment of breast cancer. Although total mastectomy provides local control and long-term survival approaching 100%, the move to breast conservation with early invasive breast cancer has forced a re-evaluation of the treatment of in-situ breast cancer. Recent advances in the evaluation and subclassification of DCIS according to histological subgroupings and sizings have provided valuable insight into the biology of the disease. These biological parameters may help to identify those lesions amenable to breast conservation. In properly selected patients, breast conservation affords a 1%/year local failure rate, with approximately one-half of the recurrences being invasive [274, 275]. DCIS of the breast represents a disease process that continues to increase in incidence with treatment paradigms that continue to evolve. Greater access to long-term data from large observational studies addressing the natural history of the disease has contributed to changes in treatment paradigms and put into question traditional management strategies. While recent analyses have suggested that a more conservative approach to the management of DCIS without surgical intervention or radiation therapy may be advisable based on breast cancer mortality data, there is a lack of level 1 or prospective evidence to support the widespread adoption of these approaches. Currently, surgery remains the standard of care for the initial treatment of DCIS. Adjuvant radiation therapy has consistently demonstrated a reduction in the risk of local recurrence following breast-conserving surgery, even in “low-risk” populations of patients. Invasive recurrences following breast-conserving surgery are associated with increases in breast cancer mortality. Surgery and radiotherapy remain standard of care treatment options in the management of DCIS. Future studies are required to identify cohorts of patients in which radiotherapy can be safely omitted as well as to evaluate whether short-course radiotherapy alone may represent a better option than endocrine therapy with respect to compliance, toxic effects, cost, and local control following breast-conserving surgery [276]. DCIS accounts for approximately 20% of mammographically diagnosed breast cancers. Currently, there is a trend to consider DCIS as a lesion for which treatment deescalation is advocated to avoid overtreatment, that is, radiotherapy in addition to breastconserving surgery or even surgery at all. The long-term follow-up updates of the firstgeneration RCT comparing lumpectomy with and without radiation therapy have confirmed that radiation halves the local failure rates. However, radiotherapy is not associated with a survival benefit just as affirmed by the recently published evaluation of the Surveillance, Epidemiology, and End Results (SEER) registries database. Nevertheless, the risk of dying of breast cancer increases about factor 18 after experience Breast Cancer 61 of an invasive local recurrence. That means at least some DCIS have the potential to progress to a life-threatening disease. At the same time, none of the recently updated prospective trials that tested the outcome after excision alone in low-risk DCIS achieved a ten-year local failure rate below 10%. DCIS is not a uniform disease. Its clinical behavior is heterogeneous, but up to date no criteria are available that allow a precise identification of patients with low or very low progression risk who do not need irradiation. Therefore, excision followed by radiotherapy is still the standard of care in patients undergoing breast conservation. Promising new approaches for risk estimation have to be validated prospectively before their use in daily practice can be recommended [277, 278]. LCIS of the breast is a well-defined pathological entity that is found in about 2.5% of all specimens of the breast taken for biopsy and most commonly occurs in premenopausal females. Its diagnosis is virtually always incidental due to the absence of any clinical indication of its presence. This lesion carries a significant risk for development of subsequent invasive carcinoma which applies equally to both breasts and which appears to increase with time. The appropriate treatment of this disease remains a controversial issue. There is certainly a perception that LCIS represents the early form of a malignant process that can be cured or prevented if appropriately treated at this stage. At the very least, an understanding of this lesion holds the potential for broadening our understanding of the physiological basis of carcinoma of the breast as a whole [279]. Although recorded studies emphasize that patients with LCIS are “at risk” for the development of invasive cancer, it has not been unequivocally demonstrated whether such an event represents a persistence of cancer due to inadequate excision or a de novo lesion. In support of the latter is the contention that lobular carcinoma exhibits a propensity for multicentricity and bilaterality. The recognition that the histological types of the subsequent invasive cancers are not universally lobular invasive might also be cited in this regard. This information also bears upon views purporting a stepwise development of lobular invasive carcinoma from its in-situ analogue. Analysis fails to confirm any significant association between invasive lobular carcinoma and multicentric lesions. The diagnostic difficulty in distinguishing lobular hyperplasia from LCIS and the inadvisability of frozen sections for this purpose is noted. Although the results of some electron microscopic studies of the in-situ lesion challenge the propriety of its “pure in-situ” nature, this criticism does not appear valid from both a pathological as well as pragmatic standpoints. Various schemes have been proposed concerning the surgical management of patients with LCIS. Certain biological principles prompt consideration of segmental mastectomy and ALN sampling as an alternative, commodious form of treatment for such lesions. There does not appear to be any unique reason to invoke any different treatment regimen for lobular invasive carcinoma than has been utilized for other invasive breast cancers [280, 281]. 62 Ciro Comparetto and Franco Borruto LCIS of the breast is commonly identified as an incidental finding in breast biopsies performed because of either a mammographic abnormality or a palpable mass. Although long recognized as an entity, the significance and optimal treatment of LCIS remains controversial. Initially regarded as a preinvasive form of breast cancer analogous to DCIS, LCIS was treated by mastectomy. As evidence mounted for an equal risk of invasive carcinoma in both breasts, bilateral mastectomy was advocated by some. More recent studies suggest that LCIS is a marker for increased risk rather than a true precursor of invasive carcinoma, and this allows a more conservative approach [282]. The incidence of LCIS and invasive lobular carcinoma of the breast is increasing. Recent data suggest that LCIS is an indolent precursor for breast cancer, rather than a pure risk factor. This could imply free surgical margins become important. The risk of contralateral carcinoma and of multifocality of invasive lobular carcinoma is higher than for invasive ductal carcinoma. Therefore, the need for mastectomy, or even for preventative contralateral mastectomy is questioned. Conventional mammography or ultrasonography cannot always give useful preoperative information about the extent of lobular cancers. The value of dynamic contrast-enhanced MRI needs to be established for these patients. The risk of invasive carcinoma after LCIS is increased. Invasive carcinoma is usually located at the index point of LCIS and is of lobular histology. Dynamic contrast-enhanced MRI can be useful in the detection and preoperative staging of invasive lobular carcinoma. The risk of local recurrence is high in patients with invasive lobular carcinoma. Mastectomy and breast reconstruction could be an option in selected patients. The response to preoperative chemotherapy is worse for invasive lobular carcinoma compared with invasive ductal carcinoma, with a greater need for rescue mastectomy. LCIS and invasive lobular carcinoma are different entities from DCIS and invasive lobular carcinoma. Their biological profile should be studied further in order to make the fine tuning of treatment possible [283-285]. We can draw the following conclusions about minimal breast cancer: the concept of minimal breast cancer as a stage of cancer that is 95% curable is a valid one, if minimal breast cancer is defined by strict parameters. Both 0.5 and 1.0 cm have been defined as the upper limit of size for minimal invasive cancer. Some data indicate that 0.5 cm is the preferable dividing line and that 1-cm cancers are no longer minimal. Other data suggest that the most important factor is ALN status. One-centimeter cancers are probably 95% curable if ALN are negative. Cancers of 0.5 cm and smaller in size are probably not 95% curable if ALN are involved. CIS appears to be curable, even if ALN are involved. Minimal breast cancer should include LCIS (lobular neoplasia) and DCIS regardless of nodal status, and (tentatively) invasive carcinoma smaller than 1 cm in total diameter, if ALN are not involved. Many cases of minimal breast cancer are asymptomatic. If special screening is not used, less than 10% of women with breast cancer will be at the minimal stage when diagnosed. Screening programs can increase this ratio to as much as one third Breast Cancer 63 of patients, perhaps even more. While serious questions about cost-effectiveness of mass screening remain, screening programs appear to represent the best way of detecting minimal breast cancer. Screening programs should include careful history and physical examination, of course. It is probable that at least 50% of all minimal cancers would be missed without mammography. After a period of significant worry about the risk of radiation, opinion seems to be changing now and many authors are willing to accept the fact that mammography is of more benefit than risk for younger women. The Health Insurance Plan (HIP) study indicated that the risk/benefit ratio becomes favorable at age 50. Many authorities would now comfortably include mammography in the screening of women age 40 or older. Some authors believe that the benefits of mammography outweight the risks for patients of all ages. The legitimate worry over the risks of mammography should not obscure a very important fact [286]. The increasingly widespread implementation of breast screening programs, combined with the use of advanced imaging modalities, such as MRI, will further increase the numbers of patients diagnosed with this disease. The current standard management for non-palpable breast cancer is localized surgical excision combined with axillary staging, using SLNB in the clinically and radiologically normal axilla. Wireguided localization (WGL) during mammography is a method that was developed over 40 years ago to enable lesion localization preoperatively: this technique became the standard of care in the absence of a better alternative. Over the past 20 years, however, other technologies have been developed as alternatives to WGL in order to overcome the technical and outcome-related limitations of this technique [287, 288]. Current recommendations for surgical management of early-stage breast cancer include breastconserving surgery with postoperative irradiation. However, studies show that mastectomy is still being used by women with early-stage breast cancer. The following patient characteristics affect the surgical decision-making process in early-stage breast cancer: age, socio-economical factors, geographic area in which the patient lives, proximity to a radiation therapy center, testing for breast cancer susceptibility gene (BRCA), breast imaging, and decision aids. Of increasing importance in the decision making about treatment of women with early-stage breast cancer are the woman’s perception of having a surgical choice and the influence of that choice on postoperative quality of life [289]. Adoption of urbanized lifestyles together with changes in reproductive behavior might partly underlie the continued rise in worldwide incidence of breast cancer. Widespread mammographic screening and effective systemic therapies have led to a stage shift at presentation and mortality reductions in the past two decades. Locoregional control of the disease seems to affect long-term survival, and attention to surgical margins together with improved radiotherapy techniques could further contribute to mortality gains. Developments in oncoplastic surgery and partial breast 64 Ciro Comparetto and Franco Borruto reconstruction have improved cosmetic outcomes after breast-conservation surgery. Optimum approaches for delivering chest-wall radiotherapy in the context of immediate breast reconstruction present special challenges. Accurate methods for intraoperative assessment of SLN remain a clinical priority. Clinical trials are investigating combinatorial therapies that use novel agents targeting growth factor receptors, signal transduction pathways, and tumor angiogenesis. Gene-expression profiling offers the potential to provide accurate prognostic and predictive information, with selection of best possible therapy for individuals and avoidance of overtreatment and undertreatment of patients with conventional chemotherapy. Short-term presurgical studies in the neoadjuvant setting allow monitoring of proliferative indices, and changes in geneexpression patterns can be predictive of response to therapies and long-term outcome [290-292]. The therapy of early breast cancer has been changing during the last decennia. It requires a multidisciplinary approach and in each of these disciplines, improvements have been implemented. The result is that treatment schedules can now be adapted to specific subgroups. Early breast cancer is defined as operable disease, using the criteria set out by Haagensen. The new developments in prognostic criteria form the basis for creating subgroups for specific treatment schedules. Data on screening promises a beneficial effect of the implementation of screening in national health care programs. Important shifts are seen in treatment schedules: the place of postoperative radiotherapy after classic ablative treatment is being challenged, whereas it plays a major role in the new breast conserving therapy schedules. The data suggest that a large proportion of “operable” cases can be treated with breast conservation. They form a major part of the prospective studies in breast cancer. Improvements in reconstruction techniques, creating better cosmetic results, make reconstruction more competitive with breast-conserving therapy. The use of chemotherapy and endocrine manipulation in early breast cancer has now been clearly confirmed by the overview technique by the Peto group, thanks to all efforts of individual trialists together [293]. As understanding of the natural history of breast cancer has increased, radical mastectomy has given way to a preference for breastsparing surgery and greater reliance on radiation therapy and chemotherapy. Probably, the most important factor for selection of treatment is consultation with a multidisciplinary team skilled in the various procedures and techniques that might be efficacious. The key to successful management is selection of an appropriate course of treatment with which the patient feels comfortable [294]. Occult breast cancer presenting with ALN metastases is an unusual presentation and can be a diagnostic and therapeutic challenge. A comprehensive work-up, including high-quality mammography is required to decrease the false-negative rates. A number of other imaging methods have been proposed in the diagnostic evaluation of these women, including breast ultrasonography, color Doppler ultrasonography, breast MRI, positron Breast Cancer 65 emission tomography (PET), and scinti-mammography. Among them, MRI may be particularly helpful, since it has a high sensitivity and may enable preoperative localization of the primary, thus allowing the surgeon to perform a breast-conserving procedure. Even pathological examination of the mastectomy specimen may not disclose the primary tumor in up to one third of patients. Treatment options include surgery, radiotherapy, and watchful waiting (followed by mastectomy when a breast tumor is detected). Traditionally, occult breast cancer is treated with total mastectomy and ALND, but accumulating data suggest that primary breast irradiation following ALND may provide an equivalent survival with the advantage of breast conservation. Occult breast cancer patients are eligible for adjuvant chemotherapy and radiation as stage II/III node-positive patients would be treated. Overall, the prognosis for occult breast cancer is equivalent to or slightly better than staged counterparts with detectable primary breast tumors [295, 296]. Breast preservation has focused attention on the extent and distribution of cancer in the breast. Recent studies suggest that there is spread from the primary tumor rather than a random distribution of cancer throughout the breast. As a result, the term multicentric has largely been replaced by multifocal. Evidence that there is a geographic relationship of secondary deposits to the primary lesions opens the way to wide excision as definitive treatment for selected patients with both invasive and in-situ breast cancer [297]. Interest in the presence and management of synchronous multiple ipsilateral breast cancer has been reported since the early 1920s. The demonstration of multiple foci of breast cancer has been reported in 9-75% of breast cancer related specimens. The large difference in reported incidence is multifactorial and related to the definitions applied, mode of detection, and pathological assessment. However, RCT comparing total mastectomy and segmental mastectomy with or without radiation over many years have shown no difference in distant disease-free survival or overall survival in patients with synchronous multiple ipsilateral breast cancer compared with unifocal breast cancer [298, 299]. Conventional indications for mastectomy reflect circumstances where breastconserving therapy could compromise oncological or cosmetic outcome. Mastectomy continues to be recommended for the majority of women with multiple lesions within the same breast. Published studies have reported divergent results regarding the oncological adequacy of breast-conserving therapy in the management of multifocal or multicentric disease. Earlier studies demonstrated high rates of local recurrence for breast-conserving therapy. More recent series have found breast-conserving therapy to be comparable to mastectomy in terms of local recurrence, distant failure, and disease-free and overall survival. Few studies have adequately evaluated cosmetic outcomes following breastconserving therapy for multifocal or multicentric breast cancer. Contemporary oncoplastic techniques have extended the clinical utility of breast-conserving therapy and 66 Ciro Comparetto and Franco Borruto are of particular relevance to breast conservation in the context of multifocal or multicentric lesions. Appropriate case selection, preoperative oncological and aesthetic planning, satisfactory clearance of the surgical margins, and adjuvant radiotherapy are of paramount importance. In the absence of level 1 guidance concerning the management of women with multifocal or multicentric disease, each case requires discussion with regard to tumor- and patient-related factors in the context of the multidisciplinary team. In selected patients with multifocal or multicentric disease, breast-conserving therapy is oncologically safe and cosmetically acceptable [300-302]. The critical question is whether multicentricity makes a difference clinically and especially to women who do not have their entire breast removed. Radical mastectomy results in a severe cosmetic and functional problem for patients. According to many authors, the goal of the treatment should be the removal of breast cancer by conservative surgical techniques (lumpectomy, subcutaneous mastectomy, and quadrantectomy), using adjuvant radiotherapy and/or chemotherapy. The use of radiotherapy as primary treatment of early breast cancer has been also suggested. There is disagreement about surgical management of breast cancer. In fact, some investigators emphasize that the natural biological history of multicentric cancers has not been documented by any adequate follow-up series in women who do not have their entire breast removed. Thus far, no difference has been seen in disease-free or overall survival between groups of patients with early breast cancer treated by an alternative therapeutic procedure and patients treated by radical mastectomy [303]. An increasingly large proportion of women with unilateral breast cancer are treated with bilateral mastectomy. The rationale behind bilateral surgery is to prevent a second primary breast cancer and thereby to avoid the resultant therapy and eliminate the risk of death from contralateral breast cancer. Bilateral mastectomy has been proposed to benefit women at high risk of contralateral cancer, such as carriers of BRCA1 and BRCA2 mutations, but for women without such mutations, the decision to remove the contralateral breast is controversial. It is important to evaluate the risk of contralateral breast cancer on an individual basis, and to tailor surgical treatment accordingly. On average, the annual risk of contralateral breast cancer is approximately 0.5%, but increases to 3% in carriers of a BRCA1 or BRCA2 mutation. Risk factors for contralateral breast cancer include a young age at first diagnosis of breast cancer and a family history of breast cancer. Contralateral mastectomy has not been proven to reduce mortality from breast cancer, but the benefit of such surgery is not expected to become apparent until the second decade after treatment. An alternative to contralateral mastectomy is adjuvant hormonal therapy (such as TMX), but the extent of risk reduction is smaller (approximately 50%) compared to 95% or more for contralateral mastectomy. Bilateral mastectomy might reduce mortality in patients with unilateral breast cancer [304]. Bilateral breast cancer has a cumulative incidence of 7-20% in patients with primary operable breast cancer, and the majority of these lesions are Breast Cancer 67 metachronous. A consensus on the management of the contralateral breast has been elusive. Much of the confusion arises from the fact that there exist marked differences of opinion regarding the impact of a second primary breast cancer on the overall prognosis. The risk of developing a contralateral breast cancer is influenced by the age of the patient, the presence of in-situ disease, lobular histology of this new lesion, multicentricity, exposure to certain types of ionizing irradiation, and, possibly, family history of breast cancer. Management options include observation (clinical and mammographic surveillance), contralateral biopsy, and, rarely, prophylactic mastectomy. It is hoped that trials of breast cancer prevention, employing drugs such as TMX, will identify agents capable of abrogating the risk of contralateral breast cancer and improve the ultimate outcome [305]. The true incidence of bilateral breast cancer, both simultaneous and subsequent, is higher than older statistics indicate, and the frequency can be expected to increase as more efficient methods of detection and treatment become commonplace. Furthermore, there is a subgroup of patients who have an especially high risk for having a second primary cancer in the other breast: if such a cancer develops, it deleteriously influences the survival of the patient. A rational approach to the management of the other breast uses contralateral biopsy as an added modality for detection and reserves prophylatic mastectomy of the other breast for those patients who are at high risk for developing cancer in it [306]. Despite extensive publications reviewing contralateral breast cancer, the role of screening and preventative measures for contralateral tumors is controversial and optimal clinical management remains undefined. Retrospective studies suggest that contralateral mammographic surveillance results in the early detection of breast cancer, but no clear survival benefit has been demonstrated. Trials of adjuvant TMX in breast cancer patients have shown a reduction in the incidence of contralateral breast cancer in both pre- and post-menopausal women. In addition, breast cancer patients treated with ovarian ablation and prednisone have significantly reduced contralateral breast cancer versus controls. In patients with primary breast cancer, there is no evidence that contralateral breast biopsies or contralateral prophylactic mastectomy reduce mortality. Based on the published literature, contralateral breast surveillance in breast cancer patients reasonably includes breast self-examination, regular physical examinations, and annual mammography. In women who have no evidence of distant metastasis at the time of contralateral breast cancer diagnosis, it is recommended that the contralateral breast cancer be treated in the same manner as a first breast cancer, taking into account prior local and systemic therapy [307]. Locally advanced breast cancer represents the most advanced stage breast cancer that is still potentially curable with surgery, radiation, and systemic therapy. There is no one encompassing definition for this disease, but in general cancers of the breast are considered to be locally advanced if they are large and/or have infiltrated into adjacent 68 Ciro Comparetto and Franco Borruto tissues (the overlying skin or underlying muscles) and/or are found to have extensive loco-RNL involvement. It is not surprising, therefore, that locally advanced breast cancer can cause significant morbidity and mortality. Recent advances in our understanding of the biology of breast cancer have made it clear that locally advanced breast cancer does not represent a single clinical entity but rather a heterogeneous group of breast tumors that share a common theme of extensive loco-regional spread without overt evidence of distant metastatic disease. Despite advances in breast cancer screening and treatment, locally advanced breast cancer remains a significant global healthcare issue [308]. In locally advanced breast cancer, treatment typically includes neoadjuvant chemotherapy, surgery, and radiation therapy. Neoadjuvant chemotherapy allows in vivo assessment of primary tumor response to chemotherapy and is achieved the early control of micrometastatic disease. It also significantly improves surgical outcomes. Patients achieving pathological complete response after neoadjuvant chemotherapy have a better survival. Tumor downsizing can make breast-conserving therapy by allowing for smaller resections and improving cosmesis. Tumor downstaging with chemotherapy can allow breast-conserving surgery in patients who are initially candidate for mastectomy. SLNB is an appropriate alternative to routine staging ALND for early-stage breast cancer patients with clinically negative ALN. During the last years, there have been a number of clinical studies on effectiveness and role of SLNB in patients receiving neoadjuvant chemotherapy. The use of SLNB is an alternative approach to ALND in patients with neoadjuvant therapy [309]. By judicious selection of patients and operative procedures, the surgeon has an opportunity to improve the quality of life and to enhance disease-free survival in specific patient subgroups, despite the fact that the local breast cancer may be advanced and untreated [310, 311]. Although relative survival for breast cancer has improved in recent years, patients who present with metastatic disease have a less than 30% five-year survival. Thus, improvements in treatment for these patients have the potential to have a significant impact on outcomes. Historically, removal of the primary breast tumor has been offered to these patients only for palliation. However, there have been recent reports that removal of the primary tumor may improve survival. Although the definitive role of removal of the primary tumor in metastatic breast cancer is not settled, it is critical to understand the complexities of this debate in order to make further gains in breast cancer survivorship [312]. The intact primary in patients diagnosed with Stage IV breast cancer is generally reserved for palliative indications. Haagensen and Stout’s 1943 criteria of inoperability for breast cancer, including tumor fixation to the chest wall, ulceration, and peau d’orange, hold true. Surgery alone is unlikely to prolong life in such patients. Improvements in breast cancer screening and awareness mean fewer patients having inoperable breast cancer. The current problem is that imagining studies reveal some patients to have oligometastatic disease with an intact primary. Several challenges to Breast Cancer 69 previous dogma to never operate on Stage IV breast cancer patients except with palliative intent have arisen [313-318]. Chest wall involvement by breast cancer remains a difficult clinical challenge that may occur at the time of the primary diagnosis or later as a result of loco-regional breast cancer recurrence. A case-by-case multidisciplinary approach is strongly recommended, and a multimodality therapy should be always considered. Full-thickness resection of the chest wall can be done with acceptable morbidity and mortality, providing a good palliation and a better quality of life even to patients with poor prognosis. Moreover, in well-selected cases, chest wall resection results in loco-regional control of disease and prolongation of life [319, 320]. Inflammatory breast cancer is the most aggressive breast neoplasm and one of the most ominous solid tumors, characterized by involvement of the skin and rapid progression of the disease. Because of distinct clinical characteristics, diagnosis can usually be made on clinical grounds. Biopsy including the overlying skin may demonstrate dermal lymphatic invasion, although the absence of dermal lymphatic invasions should not deter aggressive therapy. Surgery or irradiation alone has little effect on the natural history of this disease, since lymphatic invasion and distant metastases are often present at presentation. Inflammatory breast cancer should be considered a systemic disease [321]. Management involves careful coordination of multidisciplinary modalities, including imaging, systemic chemotherapy, surgery, and radiation therapy. The use of neoadjuvant chemotherapy has contributed significantly to improvement in overall survival since the first descriptions of this entity, and has made the role of loco-regional therapy, including surgery and radiation, critical to continued improvements in this disease [322]. Of all malignancies in women, perhaps none is as lethal or as frustrating to the surgeon as inflammatory breast cancer. No significant progress in curing or controlling inflammatory breast cancer was made until the last decades, when investigators, noting the futility of local therapies, applied systemic therapies with some significant improvement in survival [323]. Significant advances in imaging, including digital mammography, high-resolution ultrasonography with Doppler capabilities, MRI, and PET-CT, have improved the diagnosis and staging of inflammatory breast cancer. There are currently no established molecular criteria for distinguishing inflammatory breast cancer from non-inflammatory breast cancer. Such criteria would be helpful for the diagnosis and development of novel targeted therapies. Combinations of neoadjuvant systemic chemotherapy, surgery, and radiation therapy have led to an improved prognosis: however, the overall five-year survival rate for patients with inflammatory breast cancer remains very low (∼30%). SLNB and skinsparing mastectomy are not recommended. Optimal management of inflammatory breast cancer requires close coordination among medical, surgical, and radiation oncologists, as well as radiologists and pathologists. There is a need to identify molecular changes that 70 Ciro Comparetto and Franco Borruto define the pathogenesis of inflammatory breast cancer to enable eradication of it with the use of specific targeted therapies [324, 325]. Solid papillary carcinomas are tumors morphologically characterized by round, welldefined nodules composed of low-grade ductal cells separated by fibrovascular cores. These tumors are rare and affect predominantly older women. Although they are considered CIS, debate and uncertainty still exist regarding their true nature, because IHC for myoepithelial cells has shown absence of myoepithelial cell layer along the epithelial-stromal interface of the tumor in many cases. Clinically, these tumors present as a palpable, centrally located mass or as bloody nipple discharge. Pathologically, solid papillary carcinomas exhibit low-grade features, and often the tumors display neuroendocrine and mucinous differentiation. In the majority of cases an associated invasive carcinoma is present, with colloid and neuroendocrine carcinomas being the most common. The pathological differential diagnosis is broad and ranges from benign to malignant lesions. The treatment for solid papillary carcinomas is surgical excision. When invasive carcinoma is not present, the prognosis is excellent [326]. Any papillary growth of the breast presents both a diagnostic and a therapeutic challenge: For each one of them a diagnosis of whether they are malignant or benign in nature is required as well as appropriate staging and suitable treatment. In the international literature, we find a considerable amount of different terms being used for papillary breast growths. As a result, pathological and clinical evaluation is somewhat problematic. Encapsulated papillary carcinoma is an interesting subgroup of breast papillary tumors. Because of its rarity, there have been only a limited number of large clinical studies that safely assess its appropriate treatment and expected outcome. However, more safe data exist in terms of prognosis – which seems to be excellent, as almost all published studies regarding these tumors have confirmed so far [327]. Paget’s disease, described by Sir James Paget in 1874, is classified as mammary and extramammary. The mammary type is rare and often associated with intraductal cancer (93-100% of cases). It is more prevalent in postmenopausal women and it appears as an eczematoid, erythematous, moist, or crusted lesion, with or without fine scaling, infiltration, and inversion of the nipple. It must be distinguished from erosive adenomatosis of the nipple, cutaneous extension of breast carcinoma, psoriasis, atopic dermatitis, contact dermatitis, chronic eczema, lactiferous ducts ectasia, Bowen’s disease, basal cell carcinoma, melanoma, and intraductal papilloma. Diagnosis is histological and prognosis and treatment depend on the type of underlying breast cancer [328]. Paget’s disease of the breast is a disorder of the NAC that, while rare, is often associated with an underlying in-situ or invasive carcinoma. It is characterized by eczematoid changes and persistent soreness or itching of the NAC. The histogenesis of Paget’s disease of the breast continues to be debated and is important when considering treatment options. Two theories have been proposed to explain the pathogenesis of Breast Cancer 71 Paget’s disease. The epidermotropic theory suggests that Paget’s cells are ductal carcinoma cells that have migrated from an underlying carcinoma of the breast parenchyma to the epidermis of the nipple. It is supported by the predominance of breast cancer markers found in Paget’s disease [329, 330]. The in-situ transformation theory has been proposed to explain the development of this disorder in patients in whom an underlying mammary carcinoma is not found or when there is an underlying carcinoma anatomically remote from the NAC. Paget’s cells are believed to arise as malignant cells in the epidermis of the nipple independent from any other pathologic process within the breast parenchyma [331, 332]. The histogenesis of Paget’s disease has been hotly debated, and only recently has epidermotropic theory become widely accepted. With the evolution of our understanding of breast cancer, it became apparent that the prognosis of Paget’s disease was more a reflection of that of the underlying carcinoma, be it intraductal or infiltrating. The current standard treatment of Paget’s disease remains mastectomy with or without ALND. In this era of breast-conserving surgery, however, there is much evidence to suggest that conservative treatment of Paget’s disease of the breast is possible for patients in whom an underlying breast cancer cannot be located [333]. Breast sarcomas are a rare group of heterogeneous mesenchymal tumors accounting for less than 1% of all breast malignancies. Primary sarcoma of the breast accounts for less than 5% of all soft-tissue sarcomas. As experience with breast sarcoma has increased, the perceived differences with other soft-tissue sarcomas has decreased. Outcome is predicated upon histological type, degree of differentiation, and tumor size. Recurrences are primarily local as an early event and distant to the lung somewhat later in the course of the disease. Owing to the rarity of the disease, current knowledge is mostly based on numerous case reports and relatively small retrospective series. Unlike epithelial breast cancer, there is no high-level evidence to support a standard of care for primary and/or adjuvant therapy. To overcome this relative shortage of data, most therapeutic strategies for breast sarcoma are extrapolated from current treatment for soft tissue sarcoma in other locations, mainly of the extremities and thoracic wall. In general, the therapeutic approach to sarcoma of the breast should be based on a multidisciplinary strategy necessitating experienced surgeons, pathologists, radiotherapists, and medical oncologists and including surgery, radiation to improve local control, and systemic chemotherapy in selected patients [334, 335]. Whether chemotherapy is indicated is primarily determined by tumor size. There is evidence that tumors larger than 5 cm are associated with an elevated risk of systemic failure and a poor prognosis. Negative surgical margins are more important for local recurrence and overall survival than is the extent of surgical resection. Thus, neoadjuvant chemotherapy should be considered in order to shrink the tumor and help obtain negative surgical margins. After surgical resection, patients with chemosensitive tumors should undergo additional adjuvant 72 Ciro Comparetto and Franco Borruto chemotherapy to treat micrometastatic disease. Patients with tumors less than 5 cm that are easily resectable should undergo complete resection to the extent required to provide negative surgical margins. Radiation therapy should be used to improve local control in cases in which the tumor is larger than 5 cm and in cases with positive surgical margins [336]. Further advances in treatment should follow the assembly of breast sarcoma patients in specific cancer networks in specialized sarcoma referral centers. The characterization of molecular pathways active in tumorogenesis of these tumors may pave the way for the application of novel therapeutic agents [337-339]. Breast carcinoma is increasingly treated by conservation therapy. This includes wide local excision and ALN clearance followed by radiotherapy to the remaining breast. Therapeutic irradiation may be complicated by several problems, including the development of other malignant tumors [340]. Postradiation vascular tumors fall into two categories: 1) postradiation cutaneous angiosarcoma, malignant vascular neoplasms with significant morbidity and mortality; and 2) atypical vascular lesions, vascular tumors that reportedly behave in a benign manner. Postradiation vascular tumors not only present a therapeutic problem for clinicians, but they present an increasingly common diagnostic dilemma for pathologists. Although first described separately 25 years ago, the relationship between postradiation cutaneous angiosarcoma and atypical vascular lesions remains controversial. It appears that, in at least some cases, angiosarcoma can arise in the context of atypical vascular lesions, suggesting that these lesions are part of a spectrum of the same disease process. This latter view point is supported by the significant clinical and histological overlap found between both tumors [341]. Breast angiosarcoma following surgery and radiotherapy for breast cancer is a rare but important clinical entity. Breast angiosarcoma remains challenging clinically, radiologically, and histologically, and thus a high index of suspicion is required in susceptible patients. Surgery is the primary treatment option and there are an increasing number of studies on the use of radiotherapy and chemotherapy, each with variable success. There have been recent reports of patients with metastatic disease responding to taxane chemotherapy, and there might be a future role for targeted agents given the expression of tyrosine kinase receptor (c-KIT) in a subset of angiosarcomas. Overall survival, however, remains poor [342, 343]. Leiomyosarcoma of the breast was an almost unknown tumor until some 40 years ago, and the few previously published cases lacked detailed information [344-346]. Breast Cancer 73 Metaplastic breast carcinoma is a rare but aggressive type of breast cancer that has been recognized as a unique pathologic entity by the WHO, characterized by various combinations of mesenchymal, adenocarcinoma, and other epithelial components. Metaplastic breast carcinoma often manifests as a large mass, with low ALN involvement and poor prognosis. Morphologically, it is characterized by the differentiation of neoplastic epithelium into squamous cells and/or mesenchymal-looking elements (squamous cells, spindle cells, cartilage or bone, etc.). It shares many similarities with invasive ductal carcinoma and benign lesions on mammography, which further complicates the diagnosis. Knowledge and treatment patterns about metaplastic breast carcinoma demographics, presentation, and tumor characteristics are very limited. In clinical practice, metaplastic breast carcinoma is usually treated based on the guidelines developed for infiltrating ductal carcinoma. The ideal treatment paradigm for metaplastic breast carcinoma is unknown due to its low incidence and pathological variability, so potential predictors of treatment efficacy need to be explored, but studies suggest that removal of the tumor and adjuvant radiation therapy has the greatest benefit [347, 348]. Primary malignant fibrous histiocytoma of the breast proper is a very rare tumor. From the limited number of cases reported so far, it seems that a rather high rate of local recurrence and lung metastasis might be expected, and that there is still uncertainty as to the most adequate treatment of the tumor. Therefore, the need for RLN dissection (RLND) appears justified [349, 350]. Cystosarcoma phyllodes constitutes only 0.3-0.9% of all breast tumors. The term “sarcoma” was initially used because of its fleshy appearance, a more modern term is phyllodes tumor. The behavior of phyllodes tumor constitutes a spectrum from benign and locally recurrent to malignant and metastatic. In a general surgical series, 6.2% of the tumors were malignant. The microscopic appearance of phyllodes tumor is that of epithelial elements and connective tissue stroma. Malignancy is determined by characteristics of the stroma. The metastatic spread of malignant phyllodes tumor is mainly hematogenous to lung, with infrequent lymphatic involvement. Compared to breast adenocarcinoma, phyllodes tumor tends to affect a younger population, follows a different clinical course, is associated with different imaging and histological findings and is managed distinctively. There may be difficulty in differentiating the phyllodes tumor from a large fibroadenoma, but the mammographer plays a key role in reviewing the clinical and imaging data in order to arrive at the correct diagnosis. Early diagnosis with proper surgical management can often cure non-metastatic phyllodes tumors. However, in rare cases where metastasis occurs, prognosis tends to be poor. Wide local excision with 2 cm margins is the treatment of choice. In 20% of both benign and malignant cases, phyllodes tumor will locally recur. There is no proven benefit of 74 Ciro Comparetto and Franco Borruto radiation or chemotherapy, although radiotherapy may be useful in selected cases [351, 352]. Primary squamous cell carcinoma (SCC) of the breast is a rare tumor that presents a unique biological behavior. Thus, it challenges the justification for routine ALND and adjuvant therapy. SCC has several unique biological characteristics: it is associated with a lower rate of lymph node metastasis at presentation (22% vs. 40-60% for infiltrating ductal carcinoma) and a significant rate of distant metastasis without lymph node involvement. ER and PR receptor levels are usually very low. Because lymph node involvement plays a lesser prognostic and therapeutic role in this disease, a more selective approach (i.e., SLNB) has been proposed. The issue of adjuvant treatment remains unresolved, owing to lack of data. Surgical and medical treatment of SCC of the breast should be tailored to fit its distinct biological characteristics. The 5-FUdoxorubicin-cisplatin (CDDP) combination may be warranted in lieu of the combinations used for infiltrating ductal carcinoma [353]. Primary small cell carcinoma of the breast is an uncommon neoplasm that accounts for less than 1% of primary breast cancers. Histologically, these tumors have striking similarities to small cell carcinoma of the lung, usually with evidence of associated DCIS with areas of ductal, lobular, or papillary differentiation. Immune-reactivity for neuroendocrine markers is documented in two thirds of cases, while 33% to 50% are positive for ER or PR. HER-2 expression has not been reported in small cell carcinoma of the breast. Because of its rarity, its biological and clinical characteristics are still not fully understood and, to date, no standard therapy has been developed. Primary small cell carcinoma of the breast differs from more common types of breast cancer in its biological features. Treatment, which may include surgery, radiotherapy, and combination chemotherapy, is based on clinical stage and the presence of metastases. Prognosis is variable and is dependent on the initial stage of disease. An improved understanding of the clinical characteristics of this tumor will result in the development of new therapeutic modalities, which would improve its prognosis [354, 355]. Subtyping of breast cancers by means of DNA microarray analyses has given rise to the new concept of the basal-like subtype: this subtype is in effect the equivalent of socalled “triple-negative” breast cancer. Basal-like breast cancer has aggressive characteristics, such as high histological grade, mutation of the p53 gene, and negative hormone receptors. It tends to occur in relatively young women and is highly correlated with suppression of BRCA1 function. The epidermal growth factor receptor (EGFR) gene is often overexpressed in this subtype [356]. Primary neuroendocrine carcinomas of the breast are extremely rare. Tumors can only be considered small cell neuroendocrine tumor of the breast if non-mammary sites are excluded. These tumors form a diagnostic and therapeutic challenge [357-359]. Breast Cancer 75 The presence of ectopic breast tissue is reported in 2-6% of the general population with most cases being located in the axillary region. Although the same pathology occurs in both eutopic and ectopic breast tissue, primary carcinoma of ectopic breast tissue has been reported only in a small number of cases. Because an overlying accessory areola or nipple is often missing and because of a general lack of awareness among physicians and patients concerning these unsuspicious nodules, clinical diagnosis is frequently delayed. Histological diagnosis can also be delayed if ectopic breast tissue is not present or screened for in the biopsy specimens as apocrine glands of the breast and skin, respectively, exhibit striking similarities and IHC is of limited help. Two thirds of reported cases of primary ectopic breast carcinoma arose within the axillae [360, 361]. Metastases to the breast from extra-mammary tumors are infrequent. The main challenge in diagnosis is differentiating them from primary breast cancer. Radiologically, this can be difficult as there are no specific imaging characteristics for metastases to the breast. Cytopathological evaluation, as well as full radiological assessment, is vital to avoid unnecessary surgery. Sources of primary tumors include a wide variety of cancers. The commonest sources of primary tumors include lymphoma, lung, ovarian and cervical carcinoma, intestinal carcinoid, and rare cases of Ewing’s sarcoma and malignant pigmented melanocytic schwannoma (low-grade malignant melanoma) [362]. With the improvements in imaging techniques that have allowed the earlier detection of smaller breast cancers and the desire for improvements in cosmetic outcome, a number of minimally invasive techniques for the treatment of early stage breast cancers are being investigated. Minimally invasive therapy has been explored as a potential means of treating breast tumors with minimal disruption to adjacent soft tissues. The purpose of this is to facilitate improved cosmesis and to offer treatment to women who are unfit for surgery. A number of treatment modalities including thermal therapies (interstitial laser photocoagulation, radiofrequency, focused ultrasound, microwave ablation, and cryotherapy), percutaneous excision, and interstitial radiotherapy are being developed. All of these techniques have shown promise in the treatment of small cancers of the breast. However, additional research is needed to determine the efficacy of these techniques when they are used as the sole therapy and to determine the long-term local recurrence rates and survival associated with these treatment strategies [363, 364]. The carbon dioxide (CO2) laser has several properties which make it advantageous for breast surgery. Experimental evidence documents a marked reduction of local tumor recurrence following surgery with lasers. Preliminary human studies suggest that laser use lengthens the disease-free interval and may decrease local recurrence. Interstitial laser therapy holds promise for use in the treatment of locally advanced breast tumors and has been suggested by some as a potential modality for the primary therapy of breast cancer. The clinical use of lasers in the treatment of breast cancer is justified [365]. 76 Ciro Comparetto and Franco Borruto It is well established that the development and homeostasis of the mammary gland are highly dependent upon the actions of ovarian hormones progesterone and estrogen, as well as the availability of PRL for the pregnant and lactating gland. More recently, it has become apparent that immune system cells and cytokines play essential roles in both mammary gland development as well as breast cancer. Hormonal control of the immune system may contribute to mammary development at each stage via cytokine secretion and recruitment of macrophages, eosinophils, mast cells, and lymphocytes. Collectively, these alterations may create an immune-tolerant or inflammatory immune environment at specific developmental stages or phases of the menstrual cycle. Of particular interest for further research is investigation of the combinatorial actions of progesterone and estrogen during the luteal phase of the menstrual cycle and key developmental points where the immune system may play an active role both in mammary development as well as in the creation of an immune-tolerant environment, thereby affecting breast cancer risk [366]. It has been claimed that the timing of surgery in relation to the menstrual cycle can significantly influence the prognosis among premenopausal women with primary operable breast cancer. Those undergoing surgery at a time of unopposed estrogen (days 3-12) had a significantly worse prognosis than those who had operations at other phases of the menstrual cycle. This was also borne-out by another study that indicated that progesterone levels at the time of surgery had prognostic significance, with node positive patients in the luteal phase (progesterone more than 1.5 ng/ml) having a significantly better outcome. A meta-analysis has shown a highly significant heterogeneity of results, which do not overall show an effect. The results are heterogeneous, and the quality of the studies is in general low. Many studies suffer from statistical problems concerning small sample sizes and subgroup analyses. In all, the scientific basis for the hypothesis seems weak [367, 368]. As genetic and biological treatment modalities are developed that can be customdesigned for individual patients, the possibility that breast cancer can be managed as a chronic long-term disease becomes more real, and the requirement for minimally invasive surgical intervention used as part of a multidisciplinary treatment approach becomes more pressing [369]. Over recent decades, breast carcinoma surgery has witnessed a considerable evolution. The extent of surgery undertaken has progressively reduced, leading to less disfigurement and a significant improvement in quality of life, thereby offering women considerable motivation to seek early diagnosis. From an oncological perspective, outcome was found to be equally effective, and this key observation provided us with significant impetus to investigate the effects of reducing the radiotherapy field. Conservative surgery of breast carcinoma, both in the breast and in the ALN, has yielded very good results in terms of overall survival and impact on quality of life [370]. Breast Cancer 77 A survey of controlled RCT of orthodox therapy directed to the breast and ALN has indicated that: 1) if radical mastectomy is performed, postoperative radiotherapy gives no advantage over a watching policy; and 2) if postoperative radical radiotherapy is given, there is no need for other than a simple mastectomy. A large multicenter trial has also indicated that simple mastectomy alone with reservation of radiotherapy for treating local recurrent disease is a safe initial treatment. These results refer to survival: postoperative radiotherapy reduces the incidence of local recurrence, but this apparently can be equally well treated when it occurs. Local excision of the tumor, followed by radiotherapy, has been reported to give inferior results to a radical approach in Stage II tumors. Recognition that all these methods of local treatment fail to cure the majority of patients has emphasized the need to define the extent of the disease and to apply treatment according to that extent. A policy of selective local therapy based on this principal in which total mastectomy is combined with biopsy of the pectoral lymph nodes and further treatment by radiotherapy given only if these nodes are involved by tumor has been studied. This policy has been compared with a standard radical approach and gave similar results [371]. Subcutaneous mastectomy appears to remain a valid procedure. A 50-year experience indicated that it is feasible in at least 80% of selected patients to successfully remove the breast parenchyma and subsequently reconstruct the breast without serious complications. It is reasonable to assume that as surgical technique and experience improve, the complication rate will diminish. Furthermore, it is essential that better breast implants be devised and developed in the future. This would clearly enhance all forms of cosmetic breast surgery. The use of subcutaneous mastectomy as a prophylactic cancer procedure will parallel the improvement of implant breast reconstruction. Whether subcutaneous mastectomy will measurably reduce the mortality rate of breast cancer, of course, will take a generation to determine. However, it is a justifiable and valuable surgical tool in the treatment of breast disease. The concept of almost total breast biopsy has great merit in the discovery of occult carcinoma. Clearly, caution should be exercised in the selection of cases for this modality, and further study must be devoted to develop diagnostic guidelines of ever-increasing precision to determine which breasts are potentially malignant and should be afforded the procedure [372]. Skin-sparing mastectomy is an oncologically safe technique in selected cases, including invasive breast cancer less than 5 cm, multicentric tumors, DCIS, and prophylactic risk-reduction surgery. The high risk of local recurrence excludes inflammatory breast cancers and tumors with extensive involvement of the skin. Skin- 78 Ciro Comparetto and Franco Borruto sparing mastectomy can facilitate immediate breast reconstruction and is associated with an excellent aesthetic result. Prior breast irradiation or the need for postmastectomy radiotherapy do not preclude skin-sparing mastectomy, however the cosmetic outcome may be affected. NAC preservation is possible for remote tumors, employing a frozen section protocol for the retroareolar tissue. There is limited data available for endoscopic mastectomy and superiority over conventional skin-sparing mastectomy has not been established. In appropriately selected cases, skin-sparing mastectomy is oncologically adequate. There are several patient-centered advantages over conventional mastectomy, including aesthetic outcome and the avoidance of multiple staged procedures [373]. Nipple-sparing mastectomy is a surgical procedure that allows the preservation of the skin and NAC in breast cancer patients or in patients with prophylactic mastectomy. However, the oncological safety and patient selection criteria associated with nipplesparing mastectomy are still under debate. The incidence of NAC involvement of breast cancer in recent studies ranges from 9.5-24.6%, which can be decreased through careful patient selection. Tumor-nipple distance, tumor size, lymph node involvement, and molecular characteristics can be evaluated preoperatively by clinical examinations, imaging studies, and biopsies to predict the risk of NAC involvement. Currently, there is no available standard protocol for surgical approaches to nipple-sparing mastectomy or pathological examination of nipple-sparing mastectomy specimens. The local recurrence (ranges from 0-24%) of nipple-sparing mastectomy is not significantly higher than that of traditional mastectomy in selected patients based on long-term follow-up. The role of radiotherapy in nipple-sparing mastectomy is still controversial and is not universally accepted. Nipple-sparing mastectomy appears to be oncologically safe following careful patient selection and assessment of margins [374, 375]. Breast-conserving therapy is the best method for breast cancer treatment when concerning the psychological sequelae to the patient, so it has become the standard of care, and survival is now excellent. Cosmetic results after conservative surgery are not always acceptable: about 20% of patients need a revision operation and correcting the residual defect of the breast or asymmetry of the breasts afterwards. Consequently, the focus of breast-conserving therapy has increasingly shifted to cosmetic outcome, quality of life, and patient satisfaction. Nonetheless, excision of certain tumors still presents a considerable challenge. Specialized approaches combining oncological surgery and plastic surgery techniques are collectively referred to as oncoplastic breast surgery. Oncoplastic surgery means that the methods familiar to plastic surgeon are used to increase the number of patients treated with conservative surgery without compromising the oncological results. Even wider margins than in normal breast conservation can be gained, if local glandular flaps, musculocutaneous latissimus dorsi flaps, or microvascular free TRAM flaps are used to immediately preserve the shape and symmetry of the breasts. With plastic surgery it is possible to reshape the breast, replace Breast Cancer 79 the nipple, and gain breast symmetry. As we know, every tenth woman will have a breast cancer during her lifetime, and 80% of breast cancer women will survive. It is important to operate breast cancer immediately to save costs and help a patient feel that her breasts are still a part of her own body. Using oncoplastic surgical techniques for breast preservation, breast surgeons can achieve widened surgical margins at the same time that the shape and appearance of the breast is preserved and sometimes rejuvenated. Oncoplastic surgical resection is designed to follow the cancer’s contour, which generally follows the segmental anatomy of the breast, which has been well understood since the mid 19th century because of pioneering anatomic studies performed by Sir Astley Paston Cooper. The quadrantectomy, developed by Veronesi and colleagues in the 1970’s, follows these same anatomic principles of wide segmental resection. The more surgically narrow lumpectomy as popularized in the USA uses a smaller, scooplike non-anatomic resection of cancer. With negative surgical margins, the lumpectomy is equivalent to the quadrantectomy in achieving the goals of breast conservation as measured by local recurrence and survival. However, the lumpectomy is less versatile for resection of larger cancers, and can be more prone to creating suboptimal cosmetic defects. Cancers with large in-situ components can be particularly problematic for resection with the standard lumpectomy, when they extend both centrally toward the nipple and peripherally to distal terminal ductulo-lobular units, which typically occur in a pie-shaped segmental distribution. Ductal segments, each of which ultimately drains to a single major lactiferous sinus at the nipple, vary in size and depth in the breast. Breast surgeons should carefully evaluate the cancer distribution and extent in the breast before operation. A combination of imaging methods (mammography with magnification views, ultrasonography, MRI, or all) may yield the best estimates of overall tumor extent. Multiple bracketing wires afford the greater help to complete surgical excision. Those tumors with segmental spreading are best excised by oncoplastic resections according to their distribution. A summary of oncoplastic breast surgery outcomes would facilitate decision-making and best treatment selection by both clinicians and patients. In these studies, 80-93% of the tumors were invasive. Tumor-free resection margins were observed in 78-93%, resulting in a 3-16% mastectomy rate. Local recurrence was observed in 0-7% of the patients. Good cosmetic outcome was obtained in 84-89% of patients. However, most studies showed significant weaknesses including lack of robust design and important methodological shortcomings, negatively influencing generalizability. Current evidence supporting the efficacy of oncoplastic breast surgery is based on poorly designed and underpowered studies. Given the increasing importance and application of oncoplastic breast surgery, there is a pressing need for robust comparative studies, including both RCT and well-designed, multicenter prospective longitudinal studies [376-381]. 80 Ciro Comparetto and Franco Borruto The management of patients with early-stage breast cancer has evolved over time, with the understanding that tumor biology, and not just disease burden, impact local control. Local control is greatly improved with systemic therapy, providing an opportunity to decrease the morbidity of local therapy in women with invasive breast cancer. In women undergoing breast-conserving therapy, which consists of lumpectomy and whole-breast irradiation (WBI), there has been a lack of consensus as to what constitutes a negative margin [382]. Team work is the key to successful breastconservation therapy. Patient education and the informed consent process should include a discussion about the importance of margin status. Specimen management is critically important to obtain the highest quality information about margins. Operating technique should avoid trauma to or disruption of the specimen surface. The specimen should be oriented for the pathologist using standard techniques including sutures, clips, or colored inks. Specimen radiography is mandatory to confirm complete resection of the target tissues and can be used to direct additional margin resections during the initial procedure. With a well-designed and oriented specimen, the pathologist can give an accurate description of the margin distance for both the invasive and in-situ components of the cancer. In most cases, decision-making about margins will be straightforward. When margins are positive, approaches include re-excision, mastectomy, or, as secondline treatment, radiotherapy with a high boost dose. When margins are negative, boost administration and its dose depend on the risk of local recurrence, which is linked to biopathological tumor features and surgical margin width. Clear margins (more than 5 mm) require no further surgical therapy. “Close” margins (1-4 mm) will remain a point of controversy because of conflicting reports from clinical series. At UAB, decision for re-excision is made on a case-by-case basis. Routinely, 2 mm is considered adequate, however, volume of disease and intraductal component are important considerations when making recommendations. Current evidence indicates that wider margins do not reduce local recurrence compared to “no tumor on ink.” Although margin status does not affect the choice of systemic therapy, it may delay the start of chemotherapy when further surgery is required [383]. Despite the widespread conservative management of breast cancer, the pathologists’ examination of these specimens is far from uniform. Pathologists sample margins differently, and even disagree on what constitutes a positive margin. This variability in the pathological examination of the lumpectomy creates tremendous problems in analyzing the existing literature on the importance of positive margins. Only by adopting a uniform system of margin assessment we can begin to critically evaluate the importance of positive margins in segmental resections of the breast [384-388]. The standard treatment for early breast cancer comprises wide local excision, SLNB or ALND, adjuvant medical treatment, and external beam radiation therapy (EBRT) to the whole breast. Many studies suggest that local control plays a crucial role in overall Breast Cancer 81 survival. The local recurrence rate is estimated to be 1% per year and varies between 47% after five years and up to 10-20% in the long-term follow-up. On the basis of low local recurrence rates, the concept of WBI comes up for discussion, and PBI is increasingly under consideration. A policy of EBRT for every patient undergoing breastconserving surgery is in fact associated with a number of practical difficulties, acute radiation side effects, and longer-term toxicity, all of which detract from the obvious benefits of EBRT. In addition, with a disease as common as early breast cancer and a treatment program typically requiring sophisticated radiation planning and many fractions of treatment, the policy of breast-conserving surgery plus EBRT has enormous resource implications within departments of oncology, greatly contributing to lengthy pretreatment delays. For all these reasons, an increasing interest developed in techniques of PBI, with an emphasis on the emerging technique of intraoperative radiotherapy (IORT), which was initially employed as a boost to the tumor bed for use in conjunction with EBRT to the whole breast. Intraoperative radiotherapy (IORT) is thus referred to as the delivery of a single high dose of irradiation directly to the tumor bed (confined target) during surgery. PBI [limited field radiation therapy, accelerated PBI (APBI)] is the irradiation exclusively confined to a breast volume, the tumor-surrounding tissue (tumor bed) either during surgery or after surgery without WBI. The advantage of a very short radiation time or the integration of the complete radiation treatment into the surgical procedure convinces at a first glance. Local recurrence rates could probably increase and furthermore give rise to distant metastases and a reduction in overall survival. The combination of IORT in boost modality and WBI has the ability to reduce local recurrence rates, and overall survival figures have improved during the past decades, with the advent of more effective systemic endocrine- and chemotherapy [389, 390]. IORT, in addition to WBI, has yielded excellent long-term results. Under the assumption that the majority of in-breast tumor recurrences occur in the originally affected site, APBI as the sole treatment modality was initiated in several studies and with different techniques, one of which was IORT first with electrons, later also with conventional X-rays. The question whether and for whom the gold standard of may be replaced by APBI – especially IORT – alone has recently been one of the most controversial issues of adjuvant therapy for breast cancer. Two recently published studies by Veronesi et al. and Vaidya et al. presenting short-term results of single shot IORT with electrons (ELIOT) and with an orthovoltage system (TARGIT), respectively, have further invigorated this discussion [391]. APBI is increasingly used in the hope of increasing convenience, decreasing sequelae, and maintaining cure rates. ELIOT is an attractive APBI technique because collimator placement is under the direct control of the surgeon who removes the tumor, the skin is spared, shielding protects the chest wall, and complete irradiation can be given in a single intraoperative session (avoiding five-seven weeks of WBI). ELIOT seems as safe as WBI, however, long-term results on local 82 Ciro Comparetto and Franco Borruto control and survival are not available yet [392]. Potential future directions of IORT include novel IORT techniques utilizing intraoperative brachytherapy with in-room imaging and rapid treatment planning [393]. The rapid patient accrual observed in the European breast IORT studies reported since 2000 indicates that surgeons, radiation oncologists, and women who have breast cancer are no longer content to continue to travel down the well-worn path of disfiguring ablative treatment. Breast conservation is currently viewed as the preferred mode of therapy for early-stage breast cancer in most clinical situations. Determination of the optimal combination of whole-breast EBRT and localized IORT, for dose and fractionation, is a critical issue that only recently has been addressed. Clearly, such clinical investigative endeavors should be regarded as high priority. The very low incidence of local in-breast recurrence of cancer to date suggests that another avenue for investigation might be the determination of the extent to which the lumpectomy procedure can be safely minimized when used in conjunction with IORT. Another question remaining to be addressed pertains to the utility of IORT in the management of in-breast recurrence of cancer following conservative therapy. The incidence of local failure after organ-conserving treatment is generally reported to be approximately 5-10%. Currently, the preferred mode of salvage therapy in such a clinical situation is mastectomy. The proven efficacy of IORT concurrent with lumpectomy in the primary treatment of early-stage breast cancer suggests that even local recurrences following conventional conservative treatment might be dealt with effectively and expeditiously by means of local excision plus IORT. Such treatment, if safe and effective, could prove to be much less disfiguring than mastectomy. Because breast irradiation routinely produces a desmoplastic tissue response in the breast, there seems to be an opportunity here to address local recurrences of breast cancer with local surgical extirpation enhanced by IORT. The remarkably low incidence of local recurrence of breast malignancy observed in every breast IORT study reported to date may portend an important advancement in physicians’ ability to better achieve local control of mammary carcinoma. It is hoped that such a putative improvement in the local control of breast cancer will soon translate into improved patient survival rates for this common malignancy [394-398]. With widespread use of mammography for breast cancer screening, the number of surgical procedures has also increased. Overlapping with radiographic signs of malignancy, including masses, areas of asymmetric density and architectural distortion, microcalcifications, and skin thickening, postsurgical changes may make mammographic evaluation difficult. After tumor excision and irradiation where breast alterations are more profound and prolonged, the task of distinguishing recurrent tumor from scarring or fat necrosis is even more challenging. Mammograms after breast conservation therapy for carcinoma or after cosmetic surgery require correlation with physical findings and the surgical procedures that were performed. Responses of tissue to lumpectomy and Breast Cancer 83 radiation, such as breast edema and skin thickening, are most pronounced six to 12 months after treatment, gradually resolving within one to three years. Carefully tailored mammographic studies will promote the dual goal of early detection of local tumor recurrence and avoidance of misinterpreting postoperative and irradiation changes as malignancy. Sequential examinations should begin with a postoperative preradiation mammogram for residual carcinoma, particularly when microcalcifications have been present, followed by the baseline postradiation examination at six months with the next study six months later (one year after initial treatment). Mammograms of the treated breast may be performed at intervals of six months until radiographic stability has been recognized. The contralateral, unaffected breast should be evaluated mammographically according to screening guidelines or clinical concerns. Mammograms performed after cosmetic and reconstructive procedures should be correlated with the surgical techniques and clinical history. Modified views for silicone implants can maximize visualization of breast parenchyma. Ultrasonography is a useful complement to mammography in demonstrating the origin of a palpable mass either within the implant or the breast parenchyma. In reduction mammoplasty, distorted architecture, parenchymal bands, tissue redistribution, and fat necrosis should be recognized. After mastectomy, myocutaneous reconstruction may be performed. Masses that develop within flap reconstructions most frequently represent fat necrosis, which, when calcifying oil cysts are seen, may have a characteristic radiographic appearance [399]. Many breast cancer patients undergo cross-sectional imaging at some point during or after treatment. Thoracic CT is an important modality performed for staging and surveillance. Thoracic CT examinations often show findings related to patients’ surgical or adjuvant treatment. The postsurgical changes visible on thoracic CT may include those related to lumpectomy, mastectomy, breast reconstruction, and ALN surgery. Postsurgical complications may also be seen, including fluid collections, infection, fat necrosis, and lymphedema. Recognition and appropriate interpretation of the post-therapeutic spectrum of findings are important to avoid unnecessary diagnostic imaging and minimize patient anxiety [400]. Chapter V Imaging-Guided Breast Surgery Achieving the maximum yield of breast cancers detected by mammography has required particular changes in tissue handling and examination of the mammographically directed breast biopsy. This radiographic technique, increased use of breast-conserving surgical approaches for the treatment of breast cancer, more enlightened and demanding patients, and increasing medical-legal exposure have all contributed to changes in the way surgical pathologists should process and sample breast biopsy specimens [401]. The capability to provide histological diagnoses of non-palpable lesions by performance of percutaneous needle biopsy has revolutionized breast imaging in the past decades. The radiologist who performs percutaneous breast biopsies assumes an increased level of responsibility for the patient regarding patient selection, lesion selection, performance of the biopsy procedure, interpretation of results, and patient follow-up. With variable and increasingly numerous options for the biopsy of breast lesions, careful attention must be paid to the selection of patients and types of lesions for different procedures. Critical technical considerations affect whether biopsy of a lesion can be optimally performed percutaneously, and these considerations must be factored into the recommendations for patient treatment. In addition, a limited preprocedural clinical assessment of the patient will allow a safer procedure to be performed expeditiously. Most breast abnormalities classified by using the American College of Radiology (ACR) Lexicon as 4 (suggestive) or 5 (highly suggestive, likely malignant) are suitable for either percutaneous breast needle biopsy or needle localization and excisional biopsy. In general, those lesions classified as 3 (probably benign) carry a recommendation for early follow-up and not biopsy, because the likelihood of malignancy is small. A particular advantage of percutaneous biopsy is in the diagnosis of multicentric breast cancer. CNB is less invasive and less costly than surgical biopsy, and it can be used to demonstrate multicentric disease, saving the patient a two-step surgery. However, several lesions are 86 Ciro Comparetto and Franco Borruto better treated by excision than by percutaneous biopsy. Among these are architectural distortion or loosely arranged, segmental, or regional microcalcifications. For nonpalpable breast lesions visualized on mammography, sonography, or both, imagingguided localization is required for precise needle placement either for wire localization or for percutaneous breast biopsy. The selection of which modality to use for guidance depends on: 1) 2) 3) 4) 5) 6) 7) the adequacy of visualization of the lesion by the modality used; the position of the lesion; the ease of positioning the patient; the skill of the operator; the need to reduce radiation exposure; the overall patient condition; and the size of the lesion. FNAB has a high sensitivity and specificity in the diagnosis of palpable breast lesions when the procedure is properly performed and interpreted. Variable results have been achieved with FNAB of non-palpable breast lesions under imaging guidance. Three critical components are necessary to achieve reliable results by using FNAB. These include the following: 1) accuracy in needle placement; 2) skill in performance of FNAB; and 3) expert cytopathological analysis. Accurate preoperative needle localization of non-palpable breast lesions allows the radiologist to guide the surgeon performing an open biopsy and helps to ensure that the surgical procedure can be performed quickly and can be accomplished with the best possible cosmetic result for the patient. Lesions selected for needle localization and biopsy should undergo a complete tailored imaging evaluation before the needle localization is scheduled. Specimen radiography should be performed for all nonpalpable lesions. Once the lesion has been identified on specimen radiography, the radiologist can assist the pathologist in identifying the lesion microscopically by marking the lesion within the surgical specimen [402, 403]. Only 10-20% of non-palpable breast lesions are malignant, yet all suspicious lesions must undergo biopsy. For many women with breast abnormalities, needle biopsy is a less expensive, quicker, and better tolerated alternative to surgical excisional biopsy. Localization techniques that may be used during needle biopsy include clinical guidance, the grid coordinate system, ultrasound guidance, Imaging-Guided Breast Surgery 87 and stereotactic guidance. Of these four options, only stereotactic guidance permits 3-D localization of a lesion [404]. Localization of breast lesions with specialized, computerized stereotactic radiographic equipment or ultrasound imaging with subsequent use of this image to guide percutaneous core sampling of the abnormal area is less invasive, less painful, highly accurate, and less expensive than incisional breast biopsy with preoperative needle localization. These imaging-guided techniques have progressively matured in recent years, are used regularly by more and more surgeons, and are areas of clinical care and technology [405]. In particular, mammographically-guided needle biopsy is an accurate technique for the evaluation of non-palpable breast lesions that should lead to less discomfort and anxiety, fewer complications, and less cosmetic disfigurement. Moreover, this method is done on an outpatient basis with local anesthesia, and with a significant decrease in cost when compared with conventional surgical biopsy, so that it can decrease the expense of breast cancer screening programs. The use of FNAB should lead to surgery on a much more highly selected group of patients, thus producing higher true positive surgical biopsy rates for breast cancer than has been previously possible. These incentives should serve to increase compliance with screening mammography guidelines by women and their physicians. SNB of the breast is a diagnostic technique to obtain samples of a non-palpable area deemed suspicious for malignancy on mammography. This procedure, when performed with a large core biopsy needle, appears to be about as accurate as surgical biopsy [406, 407]. Nevertheless, it is likely that stereotactic FNAB will be best suited to high volume practices in which a large number of biopsies are performed and in which experienced mammographers, cytopathologists, and breast surgeons use a team approach to manage each patient [408]. SNB should not be used to biopsy lesions that would otherwise not undergo biopsy. The radiologist who performs this procedure must understand the implications of the resultant histological diagnosis and be certain that the pathological and radiological findings are concordant. The performance of this procedure by a radiologist is important for accurate positioning and needle placement in the breast, allowing accurate diagnosis, and in minimizing the number of exposures required for the procedure, thereby keeping the radiation dose to the patient at a minimum. In addition to understanding the guidance system used in this procedure, the radiologist also should understand the needles and guns used and be able to select the most appropriate method for each patient. Patients undergoing SNB should be told that, in some instances, surgical biopsy may still be necessary. They should know that major complications are rare but possible. Before initiating an SNB program, the radiologist performing SNB should develop a mechanism for surgical referral of woman who have a complication or an SNB diagnosis requiring surgery [409]. A specific pathological diagnosis, as well as determination of invasiveness, can be achieved for mass lesions. Microcalcification can also be sampled 88 Ciro Comparetto and Franco Borruto to diagnose in-situ cancer, although the use of CNB for non-comedo intraductal cancer is still evolving. The procedure demands meticulous technique to assure accurate needle placement, and careful correlation of biopsy results and patient follow-up is mandatory. Accuracy relative to surgical biopsy of non-palpable lesions has been excellent in published series [410]. Major complications, such as hemorrhage and infection, are extremely rare, although postbiopsy ecchymosis and tenderness are not unusual. Because less tissue is removed, postbiopsy cosmetic deformity does not occur. SNB is performed by triangulating the position of a breast lesion and by obtaining views angled equally off a central axis. This can be done using dedicated tables or add-on equipment. SNB has a reported accuracy of at least 90%. All lesions for which biopsy would ordinarily be recommended are amenable to stereotactic techniques, but those near the chest wall or in the axilla may be more difficult to biopsy with some equipment. Lesions characterized by calcifications are sometimes more difficult to sample. A biopsy diagnosis of ductal atypia, because of its histological heterogeneity, requires surgical excision to exclude coexistent carcinoma, which has been found in half of women at subsequent surgical excision. A CNB diagnosis of DCIS does not preclude the discovery of invasive carcinoma at surgery. In rare instances, the small tissue volume removed at stereotactic biopsy does not permit a final diagnosis to be made: this occurs most commonly when differentiating phyllodes tumor from fibroadenoma [411]. An important component of a stereotactic core breast biopsy procedure is the mammographic and pathological correlation of findings to plan optimal patient management. The level of suspicion of a mammographic lesion, the accuracy of stereotactic targeting, and the confirmation of microcalcifications in specimen radiographs for calcified lesions must be considered carefully and correlated with the histopathological findings. Meticulous operator technique is essential to provide representative samples of the mammographic abnormality. As we have said, properly performed, imaging-guided percutaneous core breast biopsy has reduced the morbidity of surgical breast biopsy and the overall cost of breast cancer diagnosis. Recent work has demonstrated that stereotactic core breast biopsy can reduce the cost of diagnosing mammographically detected breast lesions by more than 50%. At a time when health-care policy and reimbursement decisions are influenced by cost considerations, increased use of stereotactic core breast biopsy is anticipated. Meticulous attention to technique allows maximal realization of the benefits of this procedure [412, 413]. Recently, there have been a number of new devices introduced for SNB of nonpalpable, mammographically detected lesions. The vacuum-assisted core biopsy (VACB) [Minimal Invasive Breast Biopsy (MIBB), USA Surgical Corporation, Norwalk, CT, USA; Mammotome, Biopsys Medical, Cincinnati, OH, USA] obtains multiple tissue cores (11-gauge) in a circumferential manner around the biopsy probe, inserted under stereotactic guidance. It provides more complete sampling of mammographic lesions Imaging-Guided Breast Surgery 89 than the conventional 14-gauge SNB, reducing the number of unsatisfactory biopsies. The ABBI system utilizes stereotactic technique and an oscillating blade-cutting mechanism to obtain a single large diameter (5-20 mm) tissue core, with the aim of obtaining an intact lesion in its entirety for histological assessment [414]. Percutaneous core biopsy is most often used for evaluation of BI-RADS category 4 lesions, but may also be helpful in the evaluation of some BI-RADS category 5 lesions. Stereotactic guidance is particularly useful for calcifications: for masses that can be seen with ultrasound, ultrasound guidance may be preferable because of the absence of radiation and lower cost. The automated CNB is excellent for mass lesions, but directional VACB is superior for calcifications. Directional VACB may also be preferable for small lesions that may require placement of a localizing clip and lesions that are superficial or in thin breasts. The more expensive ABBI device has substantial limitations. Complete removal of the mammographic target can occur at percutaneous biopsy, and is a more frequent event when the larger tissue acquisition devices are used. Complete removal of the mammographic target does not ensure complete excision of the histological process. Epithelial displacement can occur during all breast needling procedures, but may be less frequent at directional VACB than at FNA or automated core biopsy. There is no evidence that displaced cells are of biological significance, but displaced DCIS can mimic infiltrating carcinoma. The pathologist should be aware of the findings of epithelial displacement, to avoid misdiagnosing DCIS as infiltrating ductal carcinoma. Some lesions warrant repeat biopsy or surgical excision after percutaneous core biopsy. Repeat biopsy is warranted if histological findings and imaging findings are discordant. Surgical excision is warranted for lesions yielding a percutaneous diagnosis of atypical ductal hyperplasia or possible phyllodes tumor. Controversy exists regarding the need for surgical excision after percutaneous diagnosis of radial scar, papillary lesion, atypical lobular hyperplasia, or LCIS. Follow-up is necessary if percutaneous biopsy yields benign findings concordant with imaging characteristics. Follow-up protocols vary, but all require substantial commitment of time and resources. It can be estimated that approximately one million breast biopsies will be performed every year to diagnose approximately 200,000 breast cancers. Percutaneous core biopsy may spare many of these women the need for a more deforming, invasive, and expensive surgical biopsy [415, 416]. Ultrasound provides a versatile approach for guiding biopsies and other breast interventions. The wide availability, real-time capability, technical improvements, and increasing user experience have greatly expanded the role of ultrasound-guided interventions in the diagnosis and management of breast disease [417]. The use of ultrasonographic guidance for such procedures provides a safe and effective real-time technique to sample tissue while the patient lies in a comfortable supine position. The operator who has a good understanding of the procedure will be able to obtain reliable 90 Ciro Comparetto and Franco Borruto specimens efficiently and consistently [418]. High-frequency ultrasonography offers several distinct advantages over stereotaxy and is the technique of choice for guiding the drainage of cysts and fluid collections and the biopsy of any solid mass that can be identified on sonograms. Ultrasonography also can be used for localizing such lesions preoperatively [419-422]. Pathologist-performed, ultrasound-guided FNAB is one of the frontiers of pathology. There is little training in ultrasound-guided biopsy of breast masses. To successfully perform an imaging-guided biopsy of the breast, pathologists should understand the basics of mammography and breast ultrasound. The heart of early breast cancer detection is the screening mammogram. Abnormalities detected on screening, such as masses, densities, architectural distortions, nipple retraction, skin thickening, abnormal lymph nodes, and microcalcifications, will lead to a diagnostic mammogram and/or breast ultrasound. Lesions classified as BI-RADS 4 or 5, and a few classified as 3 lesions, require biopsy. If the lesion is visible on ultrasound, ultrasoundguided FNAB and/or CNB is the procedure of choice. Suspicious lesions visible only on mammogram require stereotactic X-ray-guided biopsy [423] For breast masses, interventional breast sonography has become the standard for guiding needle biopsy, whether a fine aspiration or a core biopsy needle is used. Ultrasonography has also become the preferred method for guiding insertion of various localization devices for non-palpable masses, and ultrasonography’s intraoperative use for this purpose is expanding. Recently, ultrasonography has been used to monitor the placement of percutaneous ablation devices, such as radiofrequency ablation needle-electrodes, into breast masses, including carcinomas. Ultrasonography is not indicated for the routine evaluation of microcalcifications. However, on occasion, clusters of microcalcifications without a mass can be visualized on sonograms with sufficient clarity to undertake a ultrasonography-guided core biopsy if stereotactically-guided biopsy cannot be performed for technical reasons [424-426]. Portable ultrasound is now used in a variety of clinical settings by specialties outside of radiology. Despite increased accessibility to ultrasound, the overall performance of ultrasound by breast surgeons is consistently low. Intraoperative ultrasound performed by surgeons reduces re-excision rates in breastconserving surgery. Outpatient-based ultrasound performed by surgeons frees up the resources of radiology departments, allowing them to focus upon patients requiring more complex diagnostic and interventional procedures, such as lumpectomy, percutaneous excision of benign masses, and ablation of cancers. For surgeons to competently perform intraoperative and outpatient-based ultrasound, a period of formal ultrasound training is necessary to acquire knowledge of ultrasound skills and techniques. This should be followed by a period of mentorship and supervised training with an experienced breast radiologist. Breast surgeon-performed ultrasound is beneficial to the multidisciplinary care of breast cancer patients. To further improve multidisciplinary care, breast surgeons and radiologists should work more collaboratively to optimize imaging applications both Imaging-Guided Breast Surgery 91 in the operating theater and outpatient department. Current advances in therapeutic percutaneous techniques are of interest to both surgeons and radiologists. In future, a hybrid specialization should be considered to incorporate accreditation in both specialties for breast interventional procedures [427]. Techniques and instrumentation are now widely available that enable interventional MRI-guided preoperative needle localization and lesion marking. Minimally invasive MRI-guided core biopsy techniques have been demonstrated but remain limited for small lesions and will be facilitated by the development of biopsy instruments that can be directly visualized using MRI. MRI-guided tumor ablation is beginning to be evaluated in a few centers. It holds promise as new treatment modality in the continuing trend toward greater breast conservation in the local therapy of breast cancer. Further studies are needed to document the ability of MRI-guided ablation to control the margins of a tumor as effectively as surgery. Patients with an extensive in-situ intraductal component may pose a significant hurdle because the extent of DCIS maybe underestimated on breast MRI. Ultimately, the success of MRI-guided thermal ablation depends on the ability of MRI to map the extent of heating during the procedure, so that the procedure can be performed to achieve complete control of the tumor margins. It is unfortunate that the conventional method for MRI thermometry – the proton resonance frequency (PRF) shift method – does not work in fat or in voxels with a mix of fat and glandular tissue and, hence, has limited applicability in the breast. Other methods, including measurement of T1 and T2, are being investigated as alternatives [428]. Thus, despite the proven high sensitivity of MRI for invasive breast cancer, MRI has lagged behind mammography and sonography as an imaging modality for guiding interventional procedures because of the lack of suitable techniques. New imaging apparatuses, pulse sequences, and MRI-compatible devices are beginning to enable MRI-guided breast interventions, including preoperative lesion localization and minimally invasive biopsy. MRI-guided tumor ablation holds promise as a future therapy for breast cancer because of the ability of MRI to reveal the progress of heating and freezing, as well as the extent of ablated tissue [429, 430]. In fact, MRI imaging of the breast has high sensitivity for the detection of invasive breast cancer. However, not all enhancing lesions are malignant. A needle localization or biopsy system is necessary to differentiate falsepositive benign enhancing lesions from the true carcinomas [431]. Technical obstacles in the integration of breast MRI-minimally invasive therapy for breast tumors include accurate determination of margins, DCIS, and localization methods. Treatment methods are cryotherapy, interstitial hyperthermia, and focused ultrasound. Other subjects include the amount of minimally invasive therapy performed to date and the ethical dilemma of clinical trials [432, 433]. However, the use of breast MRI in the diagnosis, staging, and management of breast cancer is rapidly increasing. MRI has the ability to detect malignancy that is occult to physical exam, ultrasound, and mammography. These 92 Ciro Comparetto and Franco Borruto qualities necessitate methods for MRI-guided tissue sampling [434]. For the histological verification of such lesions, percutaneous MRI-guided biopsy techniques can now be offered as an alternative to open breast biopsy [435, 436]. However, the specificity of MRI is moderate. These attributes necessitate methods for MRI-guided tissue sampling to determine the histology of MRI-detected lesions [437-440]. The majority of MRIguided breast biopsies yield benign pathology. Proper radiological-pathological correlation is an integral component of MRI-guided breast biopsy. Familiarity with the spectrum of MRI findings and key histopathological features of common benign entities will enhance the radiologist’s confidence in determining concordance and lead to improved patient management recommendations [441]. Histological work-up of just MRI-detected breast lesions has become essential with increasing use of contrast-enhanced MRI. The different MRI-guided breast interventions are performed since 1990. Presently, for reasons of costs and image quality closed magnets are most widely used. The following approaches have been described: MRIguided freehand localization in supine position, stereotactic localization in supine position, and most frequently used localization in the prone position by means of a compression device that immobilizes the breast to prevent tissue shift during intervention. Only limited experience exists with interventions on open magnets. MRIguided wire localization is a well-established procedure. Recently, percutaneous vacuum biopsy of enhancing breast lesions has become possible under MRI guidance. The new system allows accurate and safe access to lesions in any location of the breast and direct check-up of representative excision by visualization of the cavity. Thus, reliable histological evaluation of lesions smaller than 10 mm is possible with this approach [442, 443]. VACB is an imaging-guided technique introduced in 1995 that is thought to be superior to 14-gauge automated-needle biopsy for the evaluation of non-palpable breast lesions. VACB can decrease the high risk and DCIS underestimate rates, but it is unclear whether it can also decrease the miss-rates of cancer [444, 445]. The vacuumassisted biopsy device was originally introduced as a diagnostic tool. However, improvement in technology (by production of larger caliber tools) has now extended the role of VACB to therapeutic procedures. VACB has an expanding role in the surgical treatment of benign breast diseases, and improvement in technology may further extend its therapeutic role to the excision of small malignant lesions [446]. Quite a number of radiologists indicate that complete removal of an imaged lesion in the breast by transdermal tissue acquisition is beneficial for the patient. Although this claim is technologically feasible with the vacuum-assisted biopsy devices and, by virtue of a similar technology of aspiration, liposuction, there is no scientific or clinical proof that the extended procedure is indeed valuable for the patient. The optimal treatment of malignant or premalignant lesions remains open surgery with the goal to obtain pathologically free margins whenever possible. Complete removal by imaging is quite Imaging-Guided Breast Surgery 93 different from complete pathological removal. Hence, VACB elimination of suspect or malignant lesions can be considered less optimal and even malpractice in many cases. In addition, there is no evidence that complete removal of benign lesions is good for the patient. When benign lesions can be considered precursors for malignancy, they should be surgically removed as for other premalignant lesions. Most benign lesions can be treated medically as they are usually dispersed in the breast and hormone-dependent. The rest of benign breast lesions need removal only to relieve the patient of psychological stress or because of symptoms. Evidence indicates furthermore that increase in cancer risk is related to the number and extent of breast interventions in the past. VACB and other large core biopsy devices remain a useful tool in the diagnosis of breast cancer but not for treatment purposes [447]. Diagnostic trends in medicine are being directed toward cellular and molecular processes, where treatment regimens are more amenable for cure. Optical imaging is capable of performing cellular and molecular imaging using the short wavelengths and spectroscopic properties of light. Diffuse optical tomography is an optical imaging technique that has been pursued as an alternative to X-ray mammography. While this technique permits non-invasive optical imaging of the whole breast, to date it is incapable of resolving features at the cellular level. Optical coherence tomography (OCT) is an emerging high-resolution biomedical imaging technology that for larger and undifferentiated cells can perform cellular-level imaging at the expense of imaging depth. OCT performs optical ranging in tissue and is analogous to ultrasound except reflections of near-infrared (NIR) light are detected rather than sound. OCT images of a well-established carcinogen-induced rat mammary tumor experimental model show strong correlation with corresponding histopathology. These results illustrate the potential of OCT for a wide range of basic research studies and for intraoperative imaging-guidance to identify foci of tumor cells within surgical margins during the treatment of breast cancer [448]. The area of breast interventions has benefited from recent advances in devices and imaging quality. As we have seen, ultrasound, MRI, stereotactic-guided vacuum-assisted, and mechanical rotating stick freeze biopsy are the preferred methods for histological diagnosis of breast lesions. Ablation techniques are available for the treatment of benign and malignant breast disease. The MammoSite (Marlborough, MA, USA) balloon catheter can be placed percutaneously for delivering high dose short term brachytherapy [449]. The integration of imaging and thermal therapy can provide a minimally invasive or even non-invasive alternative to breast surgery for small tumors. To be successful, these therapies must achieve equivalent or even greater efficacy as surgical outcomes and must demonstrate total ablation of the dominant lesion with negative margins, while sparing normal tissue beyond the target tissue. Procedures have been validated by histopathology subsequent to resection [450]. The various techniques used for 94 Ciro Comparetto and Franco Borruto percutaneous ablation of breast cancer include thermotherapy (radiofrequency ablation, laser irradiation, microwave irradiation, and insonation with high-intensity focused ultrasound waves), cryotherapy, and irreversible electroporation. The techniques used for percutaneous ablation of breast cancer raise many questions and issues that must be addressed before percutaneous ablation can be adopted for the treatment of early breast cancer [451-453]. Although percutaneous ablation techniques have some promising potential for less-invasive treatment of breast cancer, larger multicenter trials are needed to confirm their efficacy, especially in comparison with the reference standard of lumpectomy. The use of these techniques also leads to other remaining unanswered questions, including how to manage the axilla and which patients are the best candidates for these treatments [454]. Intraoperative evaluation of breast tissue has changed as newer imaging techniques and surgical approaches to the treatment of breast cancer have placed the pathologist in a pivotal role in the management of this disease. Assessment of the index lesion and surgical margins are but two of the many tasks to face when the specimen arrives in the surgical pathology laboratory. Pathologists are also called on to correlate changes in the mammogram with the gross pathology, particularly in those cases in which the lesion is non-palpable. Careful assessment of the gross specimen coupled with prudent utilization of frozen sections is pivotal to reducing intraoperative error rates and preventing needless anxiety for the patient [455, 456]. In breast-conserving surgery, the tumor should be removed with a clean margin, a rim of healthy tissue surrounding. Failure to achieve clean margins in the initial surgery results in a re-excision procedure. Reexcision rates are reported as being 11-46% for invasive carcinoma and DCIS. Reexcisions can have negative consequences such as increased postoperative infections, negative impact on cosmesis, patient anxiety, and increased medical costs. Therefore, the surgical margin of invasive and intraductal (DCIS) breast tissue is a subject of intense discussion. Different options for intraoperative assessment are available, but all in all, they are unsatisfying. Frozen section margin examination is possible but is timeconsuming and restricted to the assessment of invasive carcinoma. In the case of DCIS, there is no procedure for intraoperative margin assessment. Thus, a solution for efficient intraoperative surgical margin assessment is needed. For this purpose, an innovative, real-time, intraoperative margin-assessment device (MarginProbe, Dune Medical Devices, Caesarea, Israel) was designed, and recent published clinical data reported a reduction of re-excisions by more than 50% [457-460]. Also several other techniques such as WGL, intraoperative ultrasound-guided resection, radio-guided occult lesion localization, and radioactive seed localization have been described and applied. The two commonly used localization techniques are radio-guided occult lesion localization and WGL [461]. Imaging-Guided Breast Surgery 95 The majority of patients with non-palpable breast lesions are eligible for breastconserving surgery guided by some kind of lesion localization. The current standard is WGL even though it has several disadvantages, the most important one being the considerable proportion of patients with insufficient resection margin. These patients require a reoperation. New methods in the field of radio-guided surgery have been developed, including radio-guided occult lesion localization (ROLL) and radioactive seed localization (RSL). Especially RSL is a very promising technique. Guided by ultrasound, a small titanium seed containing typically 1-10 megabecquerels (MBq) of radioactive iodine-125 (125I) is placed in the center of the non-palpable breast lesion. During the operation, the seed is located with a hand-held gamma probe. To date, only few cohort studies exist on the feasibility of RSL, and the method has only been tested in one RCT. The results are either equal to or superior to those obtained with WGL, with regards to achieving free margins and low reoperation rates. Additionally, the RSL technique is less unpleasant for the patient and more flexible regarding preoperative logistics. The seed can be placed a few days before surgery, in contrast to the wire used in WGL, which has to be placed within few hours of surgery. RSL has quickly become popular in surgical and radiological teams that have used the technique and will probably become an important tool for preoperative localization of non-palpable breast lesions in the near future [462]. ROLL is a possible alternative to the commonly used WGL of nonpalpable breast lesions. Intratumoral injection of a radiotracer identifies both the primary tumor and the SLN for intraoperative gamma probe-guided dissection. The studies that combined ROLL and the SLN procedure mentioned high percentages of radically excised specimens ranging from 90-95% and an identification rate of SLN up to 100%. ROLL seems a promising technique, which appears to be more radical than WGL. Localization tends to be more accurate and faster, the excision procedure is more elegant and simple to perform, and the cosmetic result seems to be better [463, 464]. A variety of optical techniques utilizing NIR light are being proposed for intraoperative breast tumor margin assessment. Immediately following a lumpectomy excision, the margins are inked, which preserves the orientation of the specimen but prevents optical interrogation of the tissue margins. A workflow allows for both NIR optical assessment following full specimen marking using molecular dyes which have negligible absorption and scattering in the NIR. The effect of standard surgical inks in contrast to molecular dyes for a NIR signal is shown. Further, the workflow is demonstrated with full specimen intraoperative imaging on all margins directly after the lumpectomy has been excised and completely marked [465, 466]. Intraoperative breast margin assessment techniques have failed to penetrate routine practice due to limitations, including slow reporting times, technical demands, and logistics. Nevertheless, emerging intraoperative margin assessment technologies are being developed to reduce positive margin and re-excision rates. Pooled data suggest that frozen section and cytology have the greatest diagnostic accuracy. 96 Ciro Comparetto and Franco Borruto However, these methods are resource intensive and turnaround times for results have prevented widespread international adoption. Emerging technologies need to compete with the diagnostic accuracy of existing techniques while offering advantages in terms of speed, cost, and reliability [467]. In summary, the institution of screening mammography protocols has increased the number of suspicious breast abnormalities requiring diagnostic intervention. Up to 80% of these lesions are benign, forcing the medical community to devise minimally invasive techniques for tissue sampling. A reduction in the number of needle-localized open breast biopsies reduces the morbidity and cost associated with imaging-detected breast masses. Ultrasound, stereotaxis, and MRI are excellent modalities for detection of breast cancers. Imaging-guided, large-CNB systems have been developed for each of these imaging modalities, enabling successful and accurate tissue sampling and, ultimately, diagnosis of a suspicious lesion. Care must be taken to ensure correlation between imaging findings and pathological diagnosis: if the two are discordant, further investigation is mandatory. There remains a role for needle-localized open breast biopsy, although is has been reduced significantly. Some patients prefer this method of diagnosis, and in others further investigation is required because of discordant findings. When the documented pathology of the breast abnormality is atypical ductal hyperplasia, atypical lobular hyperplasia, or LCIS, the patient should undergo surgical excision because of the possibility of DCIS or invasive disease in the same area. Some lesions are inaccessible with the current imaging modalities and biopsy systems available [468]. The aim of the breast team is to obtain a definitive, non-operative diagnosis of all potential breast abnormalities in a timely and cost-effective way. Percutaneous needle biopsy with its high sensitivity and specificity should now be standard practice, removing the need for open surgical biopsy or frozen section. For patients with cancer, needle biopsy provides a cost-effective and rapid way of providing not only a definitive diagnosis but prognostic information, allowing prompt discussion of treatment options, be they surgical or medical. Early removal of uncertainty also allows better psychosocial adjustment to the disease. Patients with benign conditions found either by themselves or as a result of population or opportunistic screening can be promptly reassured and discharged, removing the health care and psychological costs of surgical biopsy or repeated follow-up. Radiologists involved in breast imaging should ensure that they have the necessary skills to carry out core biopsy and/or FNA under all forms of imaging guidance. Minimally invasive, imaging-guided biopsy for breast masses promises to continue to evolve, enabling physicians to diagnose breast cancer with a high degree of accuracy without significant morbidity [469-471]. Chapter VI Breast Lymphadenectomy Enlargement of breast RLN can be due to a variety of benign and malignant causes. The most common malignant cause is invasive ductal carcinoma, which is usually visualized with mammography. Excluding breast cancer, other causes of abnormal lymph nodes that produce a negative mammogram include lymphoma, metastases from other malignancies, and benign etiologies such as inflammatory processes, infectious diseases, collagen vascular diseases, and miscellaneous causes [472]. ALN status is an important prognostic indicator for women with breast cancer and ALND provides accurate information regarding nodal status. In addition, local control of axillary disease and allocation of adjuvant systemic therapy are dependent on appropriate axillary surgery. The survival benefit of an ALND remains controversial. A technique of complete axillary clearance includes levels I, II, and III. In the axilla, the lymphatic system usually first drains into a group of low ALN (level I). The validity, as a staging procedure, of a four-node axillary sample was demonstrated 40 years ago by Prof. Sir Patrick Forrest [473]. This technique is associated with no additional morbidity to patients and incurs minimal prolongation of operative time compared with a level II dissection. Other operative descriptions of axillary surgery generally do not adequately describe a method that clearly and consistently identifies the boundaries, anatomical landmarks, and neurovascular structures that traverse the axilla. This technique, with relative ease, allows the identification and preservation of these structures in their original anatomical planes and avoids the division of the pectoralis minor muscle. The assumption that routine level III axillary clearance, as opposed to level I or level II dissection, is associated with greater morbidity warrants further evaluation [474]. The word “sentinel” is defined in The Oxford English Dictionary as “a guard, one who keeps watch or a sentry.” When translated to the concept of a tumor and its lymph node drainage, the SLN 98 Ciro Comparetto and Franco Borruto could be interpreted to mean the lymph node that guards or keeps watch over a tumor. The SLN can thus be defined as the first lymph node that drains a primary tumor within the regional lymphatic basin of that tumor. We know that tumor progression in breast cancer often occurs in an orderly, progressive fashion. So, in theory, if the SLN is tumorfree, then the rest of the nodes in the lymphatic basin should also be uninvolved by the tumor [475]. The concept of SLNB in breast cancer surgery relates to the fact that the tumor drains in a logical way through the lymphatic system, from the first to upper levels. Therefore, the first lymph node met (the SLN) will most likely be the first to be affected by metastasis, and a negative SLN makes it highly unlikely that other nodes are affected. Because ALND does not improve prognosis of patients with breast cancer (being important only to stage the axilla), SLNB might replace complete ALND to stage the axilla in clinically node-negative (N0) patients. SLNB would represent a significant advantage as a minimally invasive procedure, considering that, after surgery, about 70% of patients are found to be free from metastatic disease, yet ALND can lead to significant morbidity. Furthermore, histological sampling errors can be reduced if a single SLN is assessed extensively rather than few histological sections in a high number of lymph nodes per patient. Although the pattern of lymph drainage from breast cancer can be variable, the mammary gland and the overlying skin can be considered as a biological unit in which lymphatics tend to follow the vasculature. Therefore, considering that tumor lymphatics are disorganized and relatively ineffective, subdermal and peritumoral injection of small aliquots of radiotracer, as we will see, is preferred to intratumoral administration. Technetium-99m (99mTc)-labeled colloids with most of the particles in the 100- to 200 nanometers (nm) size range would be ideal for radio-guided SLNB in breast cancer. Lympho-scintigraphy is an essential part of radio-guided SLNB because images are used to direct the surgeon to the site of the node. The SLN should have a significantly higher count than that of background (at least 10:1 intraoperatively). After removal of the SLN, the axilla must be re-examined to ensure that all radioactive sites are identified and removed for analysis. The SLN should be processed for intraoperative frozen section examination in its entirety, based on conventional histopathology and, when needed, immune staining with anticytokeratin antibody. The success rate of radioguidance in localizing the SLN in breast cancer surgery is about 94-97% in institutions where a high number of procedures are performed and approaches 99% when combined with the vital blue dye technique [476, 477]. The detection of metastases in SLN is facilitated by the, now relatively routine, enhanced histopathological examination via step-sectioning and IHC. In clinical terms, the finding of a metastatic deposit that measures between 0.2-2 mm, that is “micrometastasis” in a SLN is largely noncontroversial. However, the presence of smaller metastatic foci detected either by routine H&E stain or by cytokeratin immune-stain [less than 0.2 mm, i.e., so-called “isolated tumor cells (ITC)”] has remained problematic since the advent of the SLNB. A broad Breast Lymphadenectomy 99 morphological range of metastatic disease in SLN may be placed in the category of ITC. To facilitate the reproducible classification of the various strata of minimal metastasis in SLN, it is recommended the following: 1) the term “ITC” (in singular form) be restricted to cases that show the presence of only a single tumor cell; 2) in situations where there are multiple isolated single cells and/or cell cluster(s) present and each cluster measures less than 0.2 mm, the term “submicroscopic metastasis” be adopted and an actual count of tumor cells present may be given; and 3) restrict the use of the term micrometastasis to cases wherein the largest metastatic focus is larger than 0.2 mm but smaller than 2.0 mm [478]. Metastatic foci to SLN have been classified into macrometastasis, micrometastasis, and ITC in 2002, and the definition of ITC was modified in 2010. Clinical significance of occult SLN metastases, being mostly composed of micrometastasis and ITC, has been clarified in terms of predictive factors for non-SLN metastasis and patient prognosis by large-scale retrospective studies and prospective RCT [479]. WHO reclassification of axillary metastases into macrometastases, micrometastases, and ITC has highlighted the issues of sampling and further histological examination of the initially negative SLNB. Molecular detection of metastatic breast cancer cells in lymph nodes is now available as a commercial kit for intraoperative use and can resolve the sampling issue [480]. ITC and micrometastases represent low-volume or minimal disease in the RLN of breast cancer patients as compared to macrometastases. SLNB is a functional selection and removal of the most likely site of regional metastasis, and gives pathologists the opportunity to concentrate detection techniques on a limited number of lymph nodes. Consequently, more lesions belonging in the two mentioned staging categories are discovered in SLN. Despite some publications contradicting stochastic models of breast cancer, micrometastases seem to reflect a prognosis intermediate between the nodenegative and macrometastatic nodal status, and they also reflect a risk of non-SLN involvement slightly higher than that associated with a node-negative status. Data are more contradictory as concerns ITC [481]. Knowledge of the anatomy and physiology of the lymphatic system is helpful when considering a particular SLNB technique. The delicate balance between internal and external pressures in a lymphatic channel can be influenced by the injection volume and by massage in a negative or positive way. The narrow openings in the interendothelial junctions determine the speed of clearance of particles with a certain size, and this has implications for the timing of lympho-scintigraphy and surgery. Tracer uptake and lymph flow are highly variable and depend on a number of factors, some of which are beyond 100 Ciro Comparetto and Franco Borruto our control. The lymphatic anatomy is not completely understood despite numerous studies since the end of the 18th century. Several topics have been elucidated in more recent studies and through experience with SLNB. First, although axillary drainage is the principal lymphatic path of the breast, any drainage pattern from any quadrant of the breast can occur. Second, most lymph from the breast flows to the nodal basins with a direct course, not passing through the subareolar plexus. Another relevant point is that gentle massage encourages lymph flow and facilitates SLN detection. The optimum size and number of labeled colloid particles remain to be established. The optimum volume of the tracer also remains to be determined. But the main controversy concerns the injection site. Although the intradermal injection technique has attractive practical features, there is currently insufficient certainty that drainage of tracer injected anywhere in or underneath the skin of the breast reflects drainage from the cancer. Connections between collecting lymphatic vessels from the tumor site and the collecting vessels from the skin and subdermal lymphatics can explain the concordance between intraparenchymal and superficial injections in most patients. To determine the technique that yields the best SLN identification rate with the lowest possible false-negative rate would require a large RCT with all patients undergoing a complete lymph node dissection and evaluation of all other ALN with serial sections and IHC. Current knowledge about sensitivity is based on examination of the other ALN with H&E staining and not with IHC. In addition, a complete level I to III dissection may not have been done in all patients, and it is not certain that pathologists removed and examined all the nodes from the specimens. The proposed study seems impossible now that routine ALND has been abandoned by the larger centers around the world. Choosing the most attractive approach requires determining the aim of lymphatic mapping. A superficial injection technique may be adequate when the purpose is to spare patients without lymph node metastases in the axilla an unnecessary ALND. An intraparenchymal injection technique should be used when the additional purpose is to determine the stage as accurately as possible and to identify SLN elsewhere [482, 483]. So-called “occult” micrometastases detected by such methods have led to speculation that some may have reached the SLN by benign mechanical transport (BMT) rather than a metastatic process. Two potential modes of BMT are: lymphatic transport of epithelial cells displaced by biopsy of the primary breast tumor and by breast massage-assisted SLN localization. The biopsy techniques under most scrutiny include FNA and large-gauge CNB. The evidence implicating breast massage prior to SLN biopsy as a mode of BMT has been supported by statistical analysis. However, no method of distinguishing massage-associated cells in SLN from true occult micrometastases is available. The significance of small epithelial clusters in SLN is currently unknown. Thus, deviation from current biopsy and SLN-localizing practices is unwarranted [484]. Breast Lymphadenectomy 101 Experimental evidence suggests that RLN are important in the initiation and possibly the maintenance of tumor immunity. “Negative” nodes denote strong tumor immunity and “positive” nodes low. The latter also serve as markers of systemic disease. From histological and immunological studies, and mostly from recent clinical studies in breast cancer, the following practical recommendations are made: 1) clinically positive ALN are best eliminated by surgery; 2) resection of positive internal mammary lymph nodes (IMLN) appears to increase survival of patients with central and inner quadrant lesions, however, destruction of these nodes by irradiation, although improving local disease control, may decrease survival; and 3) negative RLN should be preserved, as they appear to prevent lymph node metastases and stimulate systemic immunity. Only a small fraction of unresected RLN harboring micrometastases will ultimately develop palpable disease, and their elimination at that late phase yields the same results as when these nodes are treated prophylactically [485]. The prime objectives of axillary surgery in the management of breast cancer are: 1) accurate staging; 2) treatment to cure; and 3) quantitative information of metastatic lymph nodes for prognostic purposes and allocation to adjuvant protocols. It is generally agreed that ALN status in potentially curable breast cancer is considered the single best predictor of outcome and the main determinant of allocation to adjuvant therapy. No physical examination, no imaging techniques, and no molecular biological markers can today replace ALN surgery for staging purposes. The objectives of ALN surgery are best obtained by carrying out a complete ALN clearance. Nonetheless, less radical surgery is generally performed by carrying out a sampling procedure with a yield of about four nodes or a partial ALND level I-II and Rotter’s nodes, without level III nodes, with at least ten nodes recovered. Understaging the axilla is detrimental to outcome and, furthermore, loco-regional tumor control is important for survival. Accuracy of staging is enhanced by IHC staining of micrometastases, which pathologists can easily perform for one to three SLN, but not for 20 to 30 nodes, using ALND procedure. Optimum methodology for performing SLN imaging is important for accurate identification of SLN [486]. ALN surgery should therefore be conducted in accordance with high professional standards [487]. 102 Ciro Comparetto and Franco Borruto Lympho-scintigraphy has allowed the detection of alternate drainage patterns to internal mammary, infraclavicular, and supraclavicular lymph nodes. Although patients are occasionally identified who have metastases to these basins but not the axilla, this information will not impact the decision for chemotherapy in most cases [488]. Metastases to the ALN, the IMLN, or both, define a group of patients at very high risk of having systemic micrometastases leading to recurrent disease and death if surgical therapy alone is used. In patients with breast cancer in whom both ALN and IMLN were examined histologically, 5-10% had IMLN metastases in the absence of ALN metastases. With the availability of effective systemic therapy that can improve the survival of patients with operable breast cancer who have lymph node metastases, information obtained from IMLN biopsies assumes practical significance. The management of IMLN in breast cancer is controversial. IMLN are frequent mostly benign incidental findings. However, they are clinically important because they can be the primary sites of metastasis and SLN. Surgical series from the 1950s showed that one third of breast cancer patients had IMLN involvement, with a higher risk in patients with medial tumors and/or positive axillary nodes. Although, like the axilla, the IMLN are a first-echelon nodal drainage site in breast cancer, the importance of their treatment has long been debated. Seminal RCT have failed to demonstrate a survival benefit from surgical IMLN dissection (IMLND), and several retrospective studies have shown that IMLN are rarely the first site of recurrence. However, the recent widespread adoption of SLNB has stimulated a critical reappraisal of such early results. Furthermore, the higher proportion of screening-detected cancers, improved imaging and techniques (i.e., lymphoscintigraphy for radioguided SLNB) make it possible to visualize lymphatic drainage to the IMLN. The virtually systematic application of adjuvant systemic and/or loco-regional radiotherapy encourages re-examination of the significance of IMLN metastases. Moreover, RCT testing the value of postmastectomy irradiation and a meta-analysis of RCT have provided high levels of evidence that loco-regional tumor control is associated with long-term survival improvements. This benefit was limited to trials that used systemic chemotherapy, which was not routinely administered in the earlier studies. However, the contribution from IMLN treatment is unclear. Lympho-scintigraphic studies have shown that a significant proportion of breast cancers have primary drainage to the IMLN, including approximately 30% of medial tumors and 15% of lateral tumors. In the few studies where IMLN biopsy was performed, 20% of sentinel IMLN were metastatic. The risk of IMLN involvement is higher in patients with medial tumors and positive ALN. IMLN metastasis has prognostic significance, as recognized by its inclusion in the American Joint Committee on Cancer (AJCC) staging criteria, and seems to have similar prognostic importance as ALN involvement. Although routine IMLN evaluation might be indicated, it has not been routinely performed, perhaps because IMLN drainage with lympho-scintigraphy is more difficult to demonstrate than axillary Breast Lymphadenectomy 103 drainage. This difference is due to technical reasons and not the absence of lymphatics to the IMLN. Recent anatomical studies have confirmed a model of breast lymphatic drainage that comprises superficial, deep, and perforating systems. The superficial system drains to the axilla, usually to a lymph node posterior to the pectoralis minor muscle. The deep system drains to the axilla and also anastomoses with the perforating system which drains to the IMLN. The perforating system does not connect with the superficial system. The prevalence of IMLN drainage tends to reflect the method of lympho-scintigraphy, where peritumoral (deep lymphatic system) injections have a much higher likelihood of IMLN drainage than subareolar or subdermal (superficial lymphatic system) injections. The fused single-photon emission CT (SPECT)/CT images represent a further technical solution to increase the identification of IMLN and consequently can significantly reduce the false-negative rate of SLNB. Before mature results from current and future RCT assessing the benefit of IMLN irradiation become available, lymphoscintigraphy and IMLN biopsy (IMLNB) may be used to guide decisions regarding systemic and loco-regional treatment. However, even in patients with visualized primary IMLN drainage, the potential benefit of treatment should be balanced against the risk of added morbidity [489-498]. Although ALND is an excellent procedure for both staging and local control, particularly in the clinically positive axilla, it has considerable morbidity and may understage a significant proportion of patients, because it will usually miss micrometastases that can occur in approximately 10% of “node-negative” patients. An increasing number of patients whose tumors are either non-invasive (DCIS), microinvasive, tubular cancers, or low-grade Stage I tumors without lympho-vascular invasion may be spared axillary surgery because the risk of ALN disease is 0-3%. Many studies, both prospective trials and large retrospective series, show that axillary radiotherapy alone provides similar local control rates to axillary dissection in patients with clinically negative axillas. Primary treatment of the axilla with radiotherapy alone, however, does not allow appropriate staging and carries the same morbidity of surgery. SLNB is being increasingly used in patients with breast cancer to provide this information. When a SLN is identified, it is equal to or better than ALND for staging the axilla and, if the node is positive, it will help select patients who should then proceed to further axillary surgery or axillary radiotherapy [499]. So, a better understanding of the loco-regional and systemic approaches to breast cancer over the past decades has altered the perspective on surgical management of the axilla. An increased awareness of the importance of early diagnosis and appropriate staging has focused further attention on the extent of resection of ALN [500]. The diagnosis of ALN metastases remains a challenge in the management of breast cancer and is a subject of controversy. Clinical node staging clearly is limited in the assessment of ALN. Axillary mammography, ultrasonography, and CT do not provide 104 Ciro Comparetto and Franco Borruto histological information. Although nuclear MRI may have considerable value in the diagnosis of ALN metastases, it does not detect micrometastases. The use of biological markers in the assessment of ALN metastases remains a subject of investigation. On the other hand, biopsy of selected ALN or tissue with examination of histology or cytology generally would not identify a significant percentage of patients with ALN involvement. SLNB, however, is useful for assessing ALN metastases [501]. Although the concept of the SLN has existed for most of the 20th century, it was only in the last decades that, developed initially for the treatment of malignant melanoma, lymphatic mapping and SLNB have been introduced into the treatment of early breast cancer. This technique will avoid the morbidity associated with more extensive axillary dissection. A wide range of different methods and materials has been employed for lymphatic mapping, but there has been little consensus on the most reliable and reproducible technique. Nuclear medicine procedures have revealed a very interesting diagnostic potential in recent years. The different approaches proposed in the literature for lymph node visualization are: 1) 2) 3) 4) imaging with gamma-emitting tumor seeking agents; radioimmune-scintigraphy intravenous (i.v.) or by the interstitial route; lympho-scintigraphy with colloids and gamma probe SLNB; and PET. At present, lympho-scintigraphy with gamma probe SLNB and fluorodeoxyglucose18 (18FDG)-PET are the nuclear medicine approaches with the best diagnostic performance. In addition, a final statement today should consider also the increasing need to carry out an economical analysis by evaluating the cost-effectiveness of the examinations [502]. Among the recently developed imaging modalities, (18FDG)-PET has in particular been applied to the study of lymph node metastases in cancer patients. Several clinical studies have been carried out to evaluate the accuracy of PET in the axillary staging of operable primary breast cancer. These studies have sometimes provided conflicting results, either supporting the possibility of using ( 18FDG)-PET to select patients who need ALND or questioning whether (18FDG)-PET can accurately assess the axillary status in primary breast cancer. Comparing ( 18FDG)-PET with SLNB in the same series of patients, the results seem to indicate that the lower sensitivity of PET is restricted to micrometastases. Of course, this limitation of PET has to be analyzed in relation to the importance of such small axillary metastases for the outcome of patients with breast cancer. The added value offered by PET in breast cancer staging in comparison with intraoperative detection of the SLN lies in the fact that (18FDG)-PET is a non-invasive procedure that allows, within a single examination, the biological characterization of breast cancer and viewing of the entire body [503]. In the gamma probe-guided method, the use of a large-sized radiotracer (particle size, 200-1000 nm) Breast Lymphadenectomy 105 may be preferred because only one or two SLN are identified. To increase the chance of finding metastases in SLN, it is desirable to make step sections with H&E staining on permanent and frozen sections. The addition of IHC may improve the accuracy of SLN diagnosis [504]. Knowledge of the important anatomical landmarks of the axilla is important in finding and accurately reporting suspicious lymph nodes. The pathological features of nodal metastases illuminate the imaging appearances of these nodes, as depicted with all modalities. Preoperative axillary ultrasound and FNAC has recently been shown to improve patient selection for SLNB [505]. Ultrasonography is the primary imaging modality for evaluating ALN. Morphological criteria, such as cortical thickening, hilar effacement, and non-hilar cortical blood flow, are more important than size criteria in the identification of metastases. Ultrasound-guided lymph node sampling, especially with CNB, is invaluable in confirming the presence of a metastasis in a suspicious node. So, axillary ultrasound has increasingly been used to determine nodal status prior to surgery. It has been shown to be a sensitive and specific modality in the detection of nodal metastases. When combined with FNA, the specificity of this modality significantly increases [506]. CNB has been shown to be equal in safety to FNA and has a significantly lower false-negative rate. MRI is also useful, with the added benefit of providing a global view of both axillae. CT and radionuclide imaging play a lesser role in imaging the axilla. Non-invasive techniques such as paramagnetic iron oxide contrastenhanced MRI may provide genuine alternatives to axillary staging and should be evaluated within clinical trials. Selective axillary surgery could then be offered based on imaging findings and for therapeutic intent. This non-operative approach would reduce morbidity further and facilitate interpretation of follow-up imaging. Modern imaging and biopsy greatly help the axillary staging of breast cancer. Super-paramagnetic iron oxide (SPIO)-enhanced MRI offers a further advance. SLNB may become redundant with SPIO-enhanced MRI [507]. SPIO-enhanced MRI for the detection of metastases in SLN localized by CT-lymphography (CT-LG) in patients with breast cancer is a useful method of detecting metastases in SLN localized by CT-LG in patients with breast cancer. Patients with clinically negative nodes may be spared even SLNB when the SLN is diagnosed as disease-free using SPIO-enhanced MRI [508]. One of the most exciting current roles of SLNB is the ability to stage patients intraoperatively, allowing a one-step ALND if the SLN contains metastatic carcinoma. Furthermore, intraoperative assessment of SLN can now be performed rapidly and accurately using the real-time GeneSearch Breast Lymph Node (BLN) Assay (Raritan, NJ, USA). Currently, intraoperative evaluation of SLN is performed using imprint cytology with or without rapid cytokeratin staining, frozen sectioning with or without rapid cytokeratin staining, scrape preparations, or some combination of these techniques [509]. Intraoperative imprint cytology (IIC) in the assessment of SLN allows immediate, 106 Ciro Comparetto and Franco Borruto cost-effective ALND. IIC diagnosis is accurate in up to 100% of grossly abnormal SLN. Despite overall low sensitivity for grossly negative SLN, the benefits of immediate complete ALND offset the increased risk of missing micrometastases or loss of ITC by performing frozen section. IIC of the lumpectomy margins is rapid, accurate, and costeffective. It allows re-excision during initial surgery if needed with better cosmetic result. It is a useful adjunct to and frequently a replacement for frozen section in many centers [510]. However, many technical problems are correlated with the intraoperative examination of the SLN and its sensitivity and specificity. In order to avoid the incidence of false-positive or false-negative intraoperative diagnoses, the examination of SLN under local anesthesia, awaiting its definitive analysis before carrying out tumorectomy and/or axillary lymphadenectomy has been proposed [511]. There are two current techniques used to identify the SLN: radiopharmaceutical, 99m Tc-sulfur colloid and isosulfan blue dye (used in the USA), and 99mTc-labeled albumin and patent blue dye, used in Europe (the labeled albumin is not FDA-approved in the USA). There is an ongoing debate over the best tracer injection technique in lymphatic mapping for breast cancer. Excision of radioactivity that remains at the injection site in the breast cancer prevents the gamma ray scatter that may hamper retrieval of a SLN. The intralesional injection technique avoids potential injection of tracer fluid across a lymphatic watershed, it enables identification of extra-axillary SLN and allows probeguided excision of non-palpable tumors [512]. There are reported side-effects from the parenteral administration of dyes, which range from minor to life-threatening in severity. There are differences between the dyes as regards their effects. Many dyes have been used for SLNB with acceptable identification rates. There are variable side-effects for each of those dyes [513]. Recently, methylene blue dye has been shown to be an efficacious and cost-effective alternative to isosulfan blue. With the increasing popularity of SLNB, the potential complications of isosulfan blue use must be appreciated. The use of isosulfan blue due for SLNB is associated with a significant number of allergic reactions, some of which are life-threatening. Because methylene blue dye has been shown to be equally effective and does not pose a serious risk of allergic reactions, it offers an improved technique above isosulfan blue dye for SLNB [514]. Furthermore, methylene blue is superior to isosulfan blue and Patent Blue V with respect to false-negative rates. In order to standardize the SLNB technique, comparative trials to determine the most effective blue dye and national guidelines are required [515]. However, blue dyes and radioactive colloids used for clinical SLN mapping are associated with a few issues such as adverse side-effects and short retention time in SLN. In recent years, nanoscale probes for non-invasive SLN mapping have received attention due to their adaptable synthesis methods, adjustable optical properties, and good biocompatibility. The aim is to understand the status of nanomaterials for SLN mapping, challenging work and potential clinical translation in the future [516]. Breast Lymphadenectomy 107 SLN tissue can be studied by systematic serial sectioning technique designed to find metastases of given diameters with specific probabilities. A procedure whereby three microsections are prepared repeatedly at intervals of 250 micrometers (mcm) appears to be practical. Two sections from each level can be examined by routine staining and the third by IHC stain. The latter is recommended particularly for infiltrating lobular carcinoma. This method will find metastases of 0.25 mm diameter with theoretical probability of one, and metastases of 0.10 mm diameter with probability of 0.46, with reasonable costs. Metastases of these sizes are consequential and worth finding on biological and clinical grounds [517]. In the actual tumor, node, and metastasis (TNM) classification, it was recently introduced a definition of a “pN0” patient based on SLNB [518]. IHC and serial sectioning as used in the SLN procedure indeed induce a shift from pN0 to pN1a (according to TNM). By the thorough pathological examination of the SLN, isolated tumor cells and micrometastases are more frequently detected. It has been proposed to classify small micrometastases (less than 0.5 mm) in a separate pN1a minimal (min) category to prevent stage migration [519]. The limited number of SLN compared with an ALND has prompted more comprehensive lymph node analysis increasing detection of micrometastases. National data show that many women previously classified node-negative are now classified minimally node-positive. As a result, our nodal classification and cancer staging have evolved to recognize the continuum of nodal tumor burden rather than a simplistic dichotomous stratification. It is quite clear that the more sections we evaluate from SLN the more metastases we identify. However, it is impractical to expect the practicing pathologist to mount, stain, and microscopically examine every section through the SLN paraffin blocks. Despite recommendations from the College of American Pathologists (CAP) and the American Society of Clinical Oncology (ASCO), heterogeneity in the approach to SLN evaluation exists. What is needed is adherence to a standardized evaluation protocol. The most important aspect of the SLN examination is careful attention to slicing the SLN no thicker than 2.0 mm and correct embedding of the slices to assure we identify all macrometastases larger than 2.0 mm. A single section from blocks prepared in this manner will identify all macrometastases present but smaller metastases will be missed. The prognostic significance of these missed micrometastases is still being evaluated as we await SLN outcome studies. In the context of the new molecular classification of breast cancer, subgroups may be identified where detection of micrometastases has clinical significance. It is critical that both clinicians and pathologists understand there is a random component to micrometastasis distribution within the 3-D paraffin tissue blocks. If we ultimately adopt more comprehensive microscopic evaluation of SLN, the candidate sampling strategies need to be carefully considered in the context of statistically valid sampling strategies [520]. Several guidelines relating to the histopathology of SLN, including the recent European Working 108 Ciro Comparetto and Franco Borruto Group for Breast Screening Pathology (EWGBSP) guidelines, advocate multilevel assessment of grossly or intraoperatively negative SLN with levels separated by a maximum of 1 mm and allow IHC in their assessment, although this latter method is not mandatory. Both methods of intraoperative evaluation are allowable. There are also minimum requirements for the reports on SLN histology. For example, the reports should include the extent of nodal involvement, the method used for their identification, and preferably the method used for investigating the SLN, even if the results are negative. These guidelines are intended to form the basis for national guidelines in European countries [521]. Radiotracer techniques play an important role in the preoperative and intraoperative localization of the SLN. Optimal localization of the SLN requires the use of both preoperative lympho-scintigraphy and intraoperative radiosensitive probes. Lymphoscintigraphy also identifies patients with lymphatic drainage to sites other than the axilla, thereby allowing more appropriate treatment and follow-up in this subset of patients. Procedures for localizing SLN require an understanding of the kinetics of the radiopharmaceuticals or other tracers used and the detection devices employed in each institution. Both surgical and nuclear medicine personnel should understand these principles, and close cooperation between surgeons, nuclear medicine physicians, and pathologists is essential for the application of SLN techniques [522]. Using radioisotope, blue dye, or both methods, experienced surgeons can successfully localize SLN in more than 90% of cases. The effects of isotope and blue dye may be additive. SLNB reliably predicts ALN status in 98% of all patients and 95% of those who are node-positive. The operation is best learned under a formalized protocol in which a backup ALND is performed to validate the technique during the surgeon’s early experience. Enhanced pathological analysis, including serial sections and IHC staining, is an essential element of the procedure [523]. The optimal technique of lymphatic mapping utilizes a combination of vital blue dye and radiolabeled colloid. In breast cancer patients, harvested SLN are evaluated more thoroughly by detailed pathological examination using serial sectioning, IHC, flow cytometry, and reverse transcriptase (RT)-PCR techniques. This allows for the detection of smaller tumor volumes and leads to more accurate staging. Lymphatic mapping has a 68-98% success rate in identifying the SLN. The false-negative rate (defined as a negative SLN while a higher node or nodes in the axilla are positive) is between 0-2%. The morbidity associated with this procedure is minimal [524, 525]. SLNB to replace axillary dissection should only be performed by surgeons and patient management teams with appropriate training and experience. Although both radiocolloid and blue dye are used together by most surgeons, and training should be in both techniques, some experienced surgeons use one or the other almost exclusively. Imaging SLN with preoperative lympho-scintigraphy effectively assures successful SLN Breast Lymphadenectomy 109 identification. However, SLN are still identified in the majority of imaging-negative patients. Given the logistics and cost required to perform preoperative lymphoscintigraphy, its routine use may not be justified. It may be valuable for surgeons in the learning phase and in obese patients who have increased risk of intraoperative failed localization. A negative preoperative lympho-scintiscan predicts inability to localize with the hand-held gamma probe. Patients with no “hot node” on the lympho-scintiscan are more likely to have failed localization using the gamma probe only. Therefore, blue dye should be used along with the gamma probe to optimize the localization rate in these patients [526]. The central problem is that it is not possible to preoperatively predict whether the SNB will be positive, and it is even more difficult to determine the likelihood of non-SLN positivity. Various histopathological features indicate increased risk of non-SLN metastasis, including size of SNB metastasis, presence of lymphovascular invasion, multifocality, number of involved SLN, and, conversely, the number of negative SLN. These features have been combined to produce predictive nomograms but, understandably, these still lack precision. Presently, the decision to avoid ALND will depend upon both the clinician and the patient’s impression of risk, but if either requires assurance that no residual axillary disease remains, a completion clearance will be required [527]. In fact, although delayed ALND is the gold standard for evaluating axillary status after identification of a positive SLN, between 40-70% of SLN-positive patients will have negative non-SLN and undergo a non-therapeutic ALND. Accurate estimates of the likelihood of additional disease in the axilla can assist decision-making about further treatment. To predict non-SLN metastases in patients with a positive SLNB, four different nomograms have been created. There are several published scoring systems that contain different parameters to estimate the rate of non-SLN metastases in SLN-positive patients. Despite having some limitations, the Memorial Sloan-Kettering Cancer Center (MSKCC) nomogram is the most validated model to predict non-SLN status accurately [528]. In addition, surgical pathologists must recognize the need to examine these small specimens with great care, using a generally adopted protocol. Imprint cytology or frozen sections may be used, followed by additional sections for light microscopy. Immune-chemical staining with cytokeratin or other techniques to identify “submicroscopic” metastasis is often used, but the results should not be used to influence clinical decisions with respect to adjuvant therapy. “Failed” SLNB implies the surgeon’s failure to identify the SLN, in which case a complete dissection is performed. A “false-negative” SLNB implies the finding of metastasis in the excised SLN by light microscopy after a negative frozen section examination. Whether a false-negative SLNB mandates completion ALND is controversial, with clinical trials currently under way to answer this question. Although SLNB was initiated to accompany breast-conserving treatment, it is equally useful in patients undergoing mastectomy. It is more difficult to perform with mastectomy. When using blue dye only, SLNB may require a separate 110 Ciro Comparetto and Franco Borruto incision because of time constraints between injection and identification of the bluestained nodes: radiocolloid usually does not. Completion ALND after false-negative SLNB is more difficult after mastectomy. SLNB is a useful procedure that may save 70% of women with clinically negative (N0) axillae and all of those with pathologically negative axillae from the morbidity of complete ALND. Ideally, the SLN should be able to be identified in more than 95% of patients, with a false-negative rate of less than 5%, that is the target set by the 2005 guidelines of the ASCO. Until these rates can be achieved consistently, however, surgeons should not abandon traditional axillary dissection [529, 530]. Complementary use of radionuclide imaging before surgery, intraoperative probe detection, and blue dye have yielded the best reported sensitivities for finding a SLN (94%). The learning curve phenomenon, which applies to the surgeon and the nuclear medicine physician, has been recognized: measures to minimize it are being implemented. Radiation exposure to operating room and pathology personnel is very low: estimates of exposure to the surgeon’s hands are 0.2% of the annual wholebody dose received by every human being from natural background and cosmic sources [531]. The optimal route of injection of radiocolloids and dye is controversial. Despite the accepted status of SLNB as the standard for axillary staging in breast cancer patients with clinically and radiologically negative axillae preoperatively, there is surprisingly still a lack of consensus on the most appropriate site of injection of radioactive tracer with or without blue dye. When comparing intraoperative SLN identification and concordance rates, it is not possible to compare “like with like” at different injection sites (deep radioactive tracer vs superficial radioactive tracer, superficial blue dye vs deep blue dye). This leads to inaccurate conclusions due to the different properties of these materials. The only way to determine the optimal injection site of radioactive tracer and blue dye for SLN identification intraoperatively and accurate concordance rates is by direct comparisons of “like with like” when it comes to injected materials at different injection sites [532]. Based on the classic definition of the breast lymphatic drainage and recently published articles addressing the issue of peritumoral and intradermal injections, a possible new and simplified approach using intradermal injection may identify the axillary SLN more quickly and reliably [533]. Intradermal injection of tracers has reported to be successful, suggesting that dermal and parenchymal lymphatics drain to the same SLN. Extra-axillary drainage is only seen after peri- or intra-tumoral injection [534]. Intradermal injection offers superior identification rates compared with peritumoral injection, with comparable false-negative rates [535]. Although controversial, current evidence suggests that subareolar or intradermal/subdermal injection will map the same axillary SLN as peritumoral injection in the vast majority of cases, is at least as successful, and is better logistically. Peritumoral, but not alternative routes, identify extra-axillary SLN, which are important in a minority of patients. It is Breast Lymphadenectomy 111 recommended that at least some of the radiocolloid be injected peritumorally to avoid missing those SLN not located in the ipsilateral axilla. Injection of the dye and a portion of radiocolloid in an intradermal/subdermal location is reasonable to take advantage of the general ease and accuracy of intradermal/subdermal injections in identifying axillary SLN [536]. A combination of peritumoral injection with radioisotopes and subdermal or subareolar injection with blue dye may result in enhanced success rates of SLN identification [537]. A complementary way forward is to analyze the primary breast cancer for molecular markers with prognostic significance with reference to the risk for metastatic capacity and thereby obtain a “biological staging” and identify those patients in need of systemic adjuvant therapy. A large number of molecular biological factors have been shown to have prognostic significance in breast cancer, e.g., c-erbB-2, p53, urokinase-type plasminogen activator (uPA), plasminogen-activator inhibitor-1 (PAI-1), and vascular endothelial growth factor (VEGF) [538]. Other parameters as molecular markers, nuclear grading, patient age, and tumor size, are not able to predict the ALN status and consequent local therapeutic approach similar to those provided by SLNB. The extent of SLN metastatic involvement, the extracapsular spread, the size of primary tumor, and peritumoral lympho-vascular infiltration are the four characteristics shown to be significant, if considered in association and not separately as predictors of the extent of axillary involvement in presence of a positive SLN. However, so far, specific studies did not confirm concordant and reproducible results. Therefore, apart from controlled studies, ALND is always required in presence of a metastatic SLN [539]. Conventional lympho-scintigraphy does not always define the exact anatomical location of a SLN. The lymphatic drainage pattern may be unusual or may not be shown at all. The recently introduced hybrid SPECT/CT imaging could help overcome these difficulties. SPECT is a tomographic version of conventional lympho-scintigraphy and the images have better contrast and resolution. When fused with the anatomical details provided by CT into one image, a meaningful surgical “roadmap” can be created. So far, there is little literature on the use of hybrid SPECT/CT in lymphatic mapping in patients with breast cancer. All studies demonstrated an improved anatomical localization by performing additional SPECT/CT: SLN outside the axilla or nodes close to the injection site were especially easier to identify. SLN were visualized in 89-100% by combined conventional imaging and SPECT/CT, with SLN depicted only by SPECT/CT in up to 14%. Limitations for SLN detection with SPECT/CT include extra time and inconvenience for the patient and additional radiation dose. SPECT/CT is a valuable tool for SLND, especially in difficult cases, when planar lympho-scintigraphy shows no SLN or unexpected lymphatic drainage. It is concluded that SPECT/CT shows the exact anatomical location of SLN, detects SLN not depicted by conventional imaging, and therefore facilitates surgical exploration. The hybrid SPECT/CT has the potential to 112 Ciro Comparetto and Franco Borruto make image fusion a routine clinical tool that improves lymphatic mapping in patients with breast cancer [540, 541]. One-step nucleic acid amplification (OSNA) is a novel method introduced for the lymph node staging of breast cancer and has been tested in multiple series. The results of this automated molecular assay based on the quantification of cytokeratin-19 (CK-19) messenger ribonucleic acid (mRNA) show a 96% concordance rate with detailed histopathology complemented with IHC when alternative slices of the same lymph node are used for the two tests. The low false-negative rate makes OSNA suitable for the intraoperative evaluation of SLN. The false-positive rate also seems very low. Most discordant cases are explainable by low volume metastases (micrometastases), which may be missing from the material submitted for one test, but not from the different part used for the other test. It is tempting to change the gold standard for comparisons between the methods, and if this is done, histology seems to come out as a weaker test for the identification of metastases. OSNA detects more low volume nodal involvement, but it is uncertain whether these require further axillary treatment, and this will be a subject for future investigations. Therefore, it is also uncertain whether the advantage of OSNA of detecting practically all metastases due to complete sampling of lymph node tissue is clinically more important than the exclusion of metastases greater than micrometastasis that can be reliably done by intraoperative microscopy followed by permanent section histology [542]. Pooled analysis of recent studies comparing OSNA with pathology indicated that OSNA is as accurate as pathology (96.3% concordance rate) and is useful for making the decision to omit axillary dissection for OSNA-negative patients [97.4% negative predictive value (NPV)]. The advantage of OSNA over pathology is that the former allows the semiquantitative evaluation of total tumor volume in the node when a whole node is examined. OSNA is expected to be a powerful tool for the estimation of risk of non-SLN metastasis and also patient prognosis, though further studies about this issue with larger numbers of patients is needed [543]. In conclusion, modern breast surgery, as the primary treatment of invasive breast carcinoma, has been evolving over the last century. Aggressive radical surgery, which included chest wall resection, complete axillary clearance, and IMLND, has slowly changed to a less aggressive approach. This has been based on an improved understanding of the biology of the disease. Over the years, prospective RCT, performed at centers all over the world, have demonstrated that ALND does not impact on the overall survival while it helps with loco-regional control of breast cancer. Its major role, at the present time, is limited to staging and prognostication, functions that are equally well served by the limited approach of a SLNB. There is no therapeutic role for extended ALND at the current time [544]. In fact, the development of the dynamic technique of intraoperative lymphatic mapping in the 1990s resulted in general acceptance of the SLN concept. Most breast cancer patients are suitable for SLNB, and the large majority Breast Lymphadenectomy 113 reported to date has had clinical stage T1-2N0 invasive breast cancers. SLNB will play a growing role in patients having prophylactic mastectomy, and in those with “high-risk” DCIS, microinvasive cancers, T3 disease, multifocal/multicentric disease and large tumors, neoadjuvant chemotherapy, and male breast cancer. SLNB for the first time makes enhanced pathological analysis of lymph nodes logistically feasible, at once allowing greater staging accuracy and less morbidity than standard methods [545-547]. Most of the initially identified potential contraindications towards the procedure, such as non-palpability, large tumor size, pregnancy, and being previously operated in the breast or axilla, have been ruled out, whereas multifocality represents an unsolved problem. There is no consensus about the best use of the technique in patients receiving neoadjuvant treatment. There is no place for SLNB in pure DCIS, but it can be used for large high-grade in-situ cancer diagnosed through CNB, especially if a mastectomy is planned. Morbidity is low, and the recurrence rates reported so far are reassuring [548]. It is ironic that the extent, morbidity, and cost of a staging procedure (ALND) is more than that of the surgical treatment of the primary tumor [549]. The SLN data in breast cancer is so convincing that SLN information has been incorporated into the new AJCC classification of breast cancer. After the revision of the AJCC staging system for breast cancer in 2002, the evaluation of IMLN and determination of micrometastases by H&E or by IHC have become increasingly important in staging of patients. The therapeutic value of additional lymph node dissection after a positive SLN for breast cancer is still controversial. Follow-up data from breast cancer patients is somewhat limited, but available information shows that patients with negative SLN fare much better. In summary, several important patterns of metastasis can be established based on the current SLN experience: 1) the earlier the breast cancer is found, the less the metastatic potential; 2) in most cases, breast cancer follows an orderly progression of metastasis to the SLN; and 3) a small subgroup of patients may develop systemic dissemination without SLN involvement. Since metastatic cancer is usually incurable, it is important for oncologists to detect and resect an early breast cancer without delay. The challenge in the future will be to dissect these different patterns of metastasis based on molecular or genetic markers. Such information will be critical to select high-risk patients for adjuvant therapy [550, 551]. The SLN concept agrees with the modern principles of surgical oncology in breast cancer, which are related to lymphatic dissection, accurate axillary study, and less traumatic surgery. After publication of many series, it has proven its capacity to correctly 114 Ciro Comparetto and Franco Borruto stage axilla and select patients who need ALND. However, the transportation of new surgical techniques from research to practice always occurs with some ethical dilemmas related to its introduction in clinical practice [552]. If a patient requests or is offered SLNB, the benefits and risks as well as what is and is not known about the procedure should be outlined. Patients should be informed of the number of SLNB performed by the surgeon and the surgeon’s success rate with the procedure, as determined by the identification of the SLN and the false-negative rate (the presence of tumor cells in the ALN when the SLNB result is negative). Before surgeons replace ALND by SLNB as the staging procedure at their institution, they should: 1) familiarize themselves with the literature on the topic and the techniques needed to perform the procedure; 2) follow a defined protocol for all three aspects of the procedure (nuclear medicine, surgery, and pathology); and 3) perform backup ALND until an acceptable success rate (as determined by the identification of the SLN and the false-negative rate) is achieved. A surgeon who performs breast cancer surgery infrequently should not perform SLNB. There is considerable argument concerning the number of SLNB cases with ALND that surgeons should perform before they are eligible on abandoning ALND in negative SLN patients. The number of procedures of the learning curve can not be fixed for all surgeons. Only surgeons in specialized breast cancer centers can succeed in meeting current recommendations with 20-30 cases. Surgeons from affiliated community hospitals will need more than 30 cases, whereas broad-based surgeons might need as many as 60 cases with their current caseload. Not all surgeons will be able to offer the procedure to their patients by the current recommendations. Women without SLN metastases should not receive ALND. Women with one to two metastatic SLN planning to undergo breast-conserving surgery with WBI should not undergo ALND (in most cases). Women with SLN metastases who will undergo mastectomy should be offered ALND. These three recommendations are based on RCT. Women with operable breast cancer and multicentric tumors, with DCIS who will undergo mastectomy, who previously underwent breast and/or axillary surgery, or who received preoperative/neoadjuvant systemic therapy may be offered SLNB. Women who have large or locally advanced invasive breast cancer (tumor size T3/T4), inflammatory breast cancer, or DCIS (when breast-conserving surgery is planned) or are pregnant should not undergo SLNB. These recommendations are based on cohort studies and/or informal consensus. In some cases, updated evidence was insufficient to update previous recommendations [553, 554]. Recent studies have shown that it is not only the absolute Breast Lymphadenectomy 115 number of involved SLN, but also the ratio of metastatic to examined SLN [or SLN ratio (SLNR)] that confers prognostic information [555]. There is no consensus regarding the strategy for managing the RLN in patients with local breast cancer recurrence. The success rate of re-SLNB after previous surgery depends on the method used for the previous axillary surgery and the number of lymph nodes harvested. Re-SLNB may be feasible even after mastectomy. A longer disease-free interval may correlate with a lower identification rate, but this finding is not definitive. Based on data regarding back-up dissection after re-SLNB, the accuracy of re-SLNB may be as good as SLNB in primary cases. Because the altered lymphatic drainage can be detected only by lympho-scintigraphy, the radioisotope method, followed by lymphoscintigraphy, should be used. There are not many reported cases of axillary recurrence after re-SLNB, and the follow-up periods have been short. Because re-SLNB cases have a wide variety of backgrounds, it is necessary to accumulate a larger number of cases and to obtain data from longer follow-up period in order to make clear recommendations [556]. The increasing use of neoadjuvant chemotherapy for operable breast cancer has raised questions about optimal local therapy for the axilla. SLNB after neoadjuvant chemotherapy in patients presenting with clinically negative nodes has an accuracy similar to upfront SLNB and reduces the need for ALND compared with SLNB prior to neoadjuvant chemotherapy. In patients presenting with node-positive disease, clinical trials demonstrate that SLNB after neoadjuvant chemotherapy is accurate when three or more SLN are obtained, but long-term outcomes are lacking. The relative importance of pre- and post-neoadjuvant chemotherapy stage in predicting risk of loco-regional recurrence remains an area of controversy. Neoadjuvant chemotherapy reduces the need for ALND, and SLNB is an accurate method of determining nodal status after neoadjuvant chemotherapy [557-565]. Chapter VII Endoscopic Breast Surgery Endoscopic surgery has been extensively used for many surgical conditions and has gained acceptance as an alternative and less invasive approach to open surgery. However, minimal access endoscopic techniques have yet to be translated into mainstream clinical practice in breast surgery. More recently, technical innovations have made it feasible to conduct endoscopic breast cancer resection, with or without breast reconstruction, through wounds inconspicuously hidden in the axilla and periareolar region. Several clinical trials have now been conducted to demonstrate technical feasibility, assess safety, and provide follow-up data regarding oncological success of endoscopic breast surgery. Initial results have demonstrated that endoscopic breast surgery is safe and technically feasible. Early data suggests that it is possible to achieve disease control with high rates of overall survival and low rates of local relapse, recurrence, and/or distant metastases [566]. Since 1992, video-assisted surgery for the breast has been developed mainly in the field of plastic surgery, notably in breast augmentation surgery. The surgical technique for mammary reconstruction using tissue expander with endoscopic approach, associated to partial detachment of the pectoralis muscle at the fourth rib and complete or nearly complete intraoperative expansion is a well-honored technique that provides satisfying aesthetic outcomes, with minimal morbidity for the patient. Nevertheless, this technique has some potential problems: 1) wound dehiscence with extrusion of the expander; 2) patient discomfort during the expansion process (weekly visits for the refill of the expander); and 3) the poor definition of the lower pole of the breast and cranial migration of the expander with excessive roundness of the upper pole. 118 Ciro Comparetto and Franco Borruto By using intraoperative tissue expansion, these drawbacks can be avoided [567]. Today, video-assisted surgery, indicating partial or total endoscopic surgery, can be performed for the treatment of both benign and malignant breast tumors to improve the cosmetic outcome. Although, in some respects, this kind of surgery for malignant tumors is still experimental, it is feasible enough for clinical use, and is expected to become one of the standard operations for breast cancer [568]. Mammoscopy seems to be a very promising technique that allows evaluation of implant integrity and documentation of the condition of breast implants in-situ. It is a useful tool in the evaluation of intercapsular conditions in the augmented and reconstructed breast. Because of the limitations of capsular space, however, there is a greater possibility of implant disruption when multiple manipulations are attempted through separate ports of entry. It is best to use the endoscope for visualization and documentation and to minimize the amount of manipulation that has to be done through other ports for fear of rupturing the implant. Endoscopy is a limited procedure best suited for documentation and evaluation rather than endoscopic surgical procedures, particularly when capsulotomy and capsulectomy can be done more readily under direct vision through a slightly larger incision [569]. The use of subcutaneous endoscopy has allowed to dissect and perform sophisticated procedures in the subcutaneous space [570]. Genetic analysis using comparative genetic hybridization has shown evidence that the majority of benign and malignant lesions of the breast, approximately 85%, arise from the epithelium of the terminal duct-lobular unit with normal cells progressing to atypia and finally to carcinoma. Although modern mammography, ultrasound, and MRI have improved diagnosis, a final pathological diagnosis currently relies on percutaneous methods of sampling breast lesions. Mammography, which is currently the main screening modality for early detection, has a low positive predictive value (PPV) of only 25%, especially in young women with very dense breasts. Therefore, new screening approaches are needed for the early detection of breast cancer in all age groups. Two related techniques of breast epithelial sampling have emerged in the past several years: ductal lavage, in which fluid-yielding nipple ducts are cannulated at their orifices and lavaged with saline while the breast is intermittently massaged, and ductoscopy, in which discharging or fluid-yielding duct orifices are dilated, intubated with a microendoscope, and the lumen directly visualized. This may aid in detection of breast masses long before they are palpable or visible via mammography. Both of these techniques have significant potential in terms of allowing the repeated sampling of ductal epithelium over time and, as such, have generated considerable enthusiasm. However, data regarding the impact of these techniques on the detection of significant breast disease is very scant. It is important at the outset of the assessment of this new technology that breast cancer clinicians and clinical researchers think carefully about the standards of evidence that need to be met regarding the benefits of these procedures before they are widely adopted Endoscopic Breast Surgery 119 [571, 572]. Mammary ductoscopy has been used as a tool to evaluate the breast for cancer for over 20 years. It allows direct visualization of the mammary ductal system where most cancers originate using submillimeter fiberoptic microendoscopes inserted through the ductal opening onto the nipple surface, and that produces sharp and clear video images and ductal washings for cytological analysis, providing a more targeted approach to the diagnosis of disease arising in the ductal system, since the lesion can be visualized and samples collected in the area of interest. Mammary ductoscopy is a useful diagnostic adjunct in patients with pathological nipple discharge. The procedure can be performed under local anesthesia in the rapid intervention and outpatient setting. Furthermore, it can reduce the number and extent of duct excision operations for pathological nipple discharge. Abnormalities can be identified successfully by mammary ductoscopy, and intraductal biopsy can be used when the tumor is a polypoid type. Ductal lavage using microcatheters is effective in identifying malignant cells in high-risk women and this has stimulated interest in exploring the role of mammary ductoscopy in breast cancer screening. There is a growing body of evidence that mammary ductoscopy combined with ductal lavage may have a role in the management of women with pathological nipple discharge, the guiding of breast conserving surgery for cancer, and the screening of high-risk women. The addition of molecular and genetic analysis of cells obtained by mammary ductoscopy are likely to enhance the use of this technique. Mammary ductoscopy techniques are safe and appear useful for detecting abnormalities in the breast. The additional molecular biological study or ductal lavage may enhance the ability to direct and limit subsequent surgery when removing the offending lesions. However, its potential use in the early detection of breast cancer, guiding breastconserving surgery for cancer, therapeutic ablation of intraductal disease, and guiding risk-reducing strategies among high-risk women requires further research and evaluation. Cytological assessment of mammary ductoscopy is highly specific but less sensitive in the detection of breast cancer. Nonetheless, a mammary ductoscopy sample from a breast with pathological nipple discharge may rarely undergo cytological review and be interpreted as consistent with malignancy, only later to undergo surgical resection demonstrating benign pathology. For this reason, pathological nipple discharge specimens interpreted as malignant on cytological review require histopathological confirmation prior to instituting therapy. Additional sample evaluation using image or molecular analysis may improve the sensitivity and specificity of mammary ductoscopy in breast cancer detection. The development of a biopsy kit that obtains adequate microbiopsy samples for histological diagnosis under direct visualization, combining mammary ductoscopy with molecular diagnostic markers and real-time optical biopsy system for the diagnosis of premalignant and early malignant disease and radiofrequency for curative ablation of intraductal lesions, will enhance the use of this technique by breast surgeons and radiologists. Further research is required to confirm these potential 120 Ciro Comparetto and Franco Borruto applications. Furthermore, the addition of molecular and genetic analysis of cells obtained by mammary ductoscopy for diagnostic markers and the emergence of newer generations of microendoscopes with optical biopsy systems for the diagnosis of premalignant and early malignant disease are likely to enhance the use of this technique [573-578]. The intraductal approach to breast cancer and premalignant lesions has now developed to yield substantial cytological samples of exfoliated cells. Standard cytology is still inadequate in sensitivity and specificity to accurately interpret the majority of samples. As techniques evolve using ductoscopic biopsy and molecular marker panels to increase accuracy of cytological interpretation, these tools will be able to unravel the breast carcinogenesis pathways. They will also offer considerable benefit in screening for premalignant changes and developing effective chemoprevention strategies [579, 580]. Recent technical development of ductoscopes and microinstruments is shifting research interest from diagnostic to interventional ductoscopy. Diagnostic ductoscopy is performed by many breast physicians worldwide. Interventional ductoscopy, however, depends on an additional working channel and a variety of microinstruments of 0.4-0.8 mm for procedures inside the breast duct. Autofluorescence ductoscopy is a new imaging technique used on an experimental base for clinical evaluation to identify intraductal lesions. Laser ductoscopy for removal of intraductal papillomas and 3-D intraductal tracking systems are future projects. Technical innovation and further miniaturization of instruments is supporting a change from diagnostic to interventional ductoscopy. A therapeutic intraductal approach as well as autofluorescence endoscopy could potentially eliminate unnecessary biopsies and offer better identification of intraductal lesions [581]. The advantage of mammary ductoscopy is that it is possible to gain direct access to the ductal system via the nipple. Direct visualization of the duct epithelium allows the operator to precisely locate intraductal lesions, enabling accurate tissue sampling and providing guidance to the surgeon during excision. The intraductal approach may also have a role in screening individuals who are at high risk of breast cancer. Finally, in spontaneous nipple discharge, as biopsy instruments improve and intraductal therapeutics, such as intraductal excision and laser ablation, become a possibility, normal or benign ductoscopic findings may help minimize surgery in selected patients. Duct endoscopes have become smaller in diameter with working channels and improved optical definition. Currently, the role of mammary ductoscopy is best defined in the management of spontaneous nipple discharge facilitating targeted surgical excision, potentially avoiding unnecessary surgery, and limiting the extent of surgical resection for benign disease. Few prospective RCT exist in the literature, and these would be crucial to validate current opinion, not only in the benign setting but also in breast oncological surgery [582]. Endoscopic Breast Surgery 121 In summary, endoscopy is a new tool in the armamentarium for surgery of the breast. Endoscopic techniques may offer decreased scarring and morbidity rates for a variety of aesthetic and reconstructive procedures. Whereas initial clinical experiences are encouraging, most endoscopic procedures of the breast remain developmental, both in technique and instrumentation. Additional development, refinement, and experience will be required to define fully the utility of endoscopic techniques [583]. Chapter VIII Prophylactic Breast Surgery Increased understanding of risk factors for breast cancer, especially the identification of genes associated with a predisposition to breast cancer, has focused attention on the issue of breast cancer prevention [584]. Multiple factors which increase a women’s breast cancer risk have been identified. These range from conditions such as LCIS, which increase risk to relatively high levels, to reproductive factors such as nulliparity, which are associated with only a small increase in risk. When determining an individual’s risk, all her potential breast cancer risk factors must be considered. In order for risk information to be meaningful to a woman, risk must be expressed as absolute risk over a defined time interval, since there is no uniform agreement on what risk level is high enough to require intervention. At present, careful follow-up or prophylactic mastectomy are the best management options available for the woman at increased risk. The efficacy of follow-up including breast self-exam, physician exams, and screening mammography for early detection of cancer in a high-risk population is known. Prophylactic mastectomy, while highly effective, does not provide complete protection from breast cancer and is more radical than the surgery done for established cancer in many cases. Which of these options is chosen by an individual woman is dependent on how much risk she is willing to assume [585]. Therefore, at present, the care of women at increased risk of developing breast cancer poses a clinical dilemma and remains an area of controversy. A number of investigators have addressed the pros and cons of prophylactic mastectomy versus close follow-up, utilizing annual mammography, semiannual or even more frequent physical examinations of the breast, and proficient monthly breast self-examinations. Recent efforts to isolate a gene (BRCA1) on chromosome 17q12-21 raise additional concerns about the management of women testing positive for BRCA1 mutations. These women are estimated to have an 85% lifetime risk of developing breast cancer. In addition, women with a strong family history opting against 124 Ciro Comparetto and Franco Borruto testing for BRCA1 mutations may express interest in surgery [586]. Five to 10% of breast cancer is attributable to the autosomal dominant inheritance of a high-risk susceptibility gene. There are a number of known inherited cancer syndromes that confer a higher risk of breast cancer. In 1994, the BRCA1 gene, which is responsible for 45% of hereditary early-onset breast cancer and for the majority of coinheritance of breast and ovarian cancer, has been cloned and, since 1996, genetic testing for these mutations has been clinically available. Another gene that confers an increased risk of breast cancer is the BRCA2 gene, which maps to the long arm of chromosome 13 by linkage analysis. Mutations in BRCA2 account for approximately 40% of hereditary early-onset breast cancer. In addition, at least 7% of breast cancer may occur in women who are heterozygous for mutations in a gene for ataxia-telangiectasia (ATM), an autosomal recessive chromosome instability syndrome. Predictive testing for some predisposing conditions is possible through indirect or direct mutation testing [587]. Molecular alterations in proto-oncogenes, tumor-suppressor genes, and genes that function in DNA damage recognition and repair are considered to be hallmarks of a carcinogenic process, including breast carcinogenesis. The functions of the BRCA proteins are not fully understood, although it is clear that they play a role in the control of transcription, regulation of the cell cycle, and management of DNA damage. Genetic risk factors and family history play an important role in breast cancer development. Inherited genetic susceptibility to breast cancer can be due both to genes which confer a high degree of risk and to polygenes which have a smaller effect on disease risk. Although a greater proportion of inherited breast/ovarian cancers are due to the BRCA1 and BRCA2 mutations, a number of new genes have emerged as susceptibility candidates, including rare germline mutations in high penetrance genes, such as p53 (LiFraumeni syndrome) and phosphatase and tensin homolog (PTEN, Cowden disease), and more frequent mutations in moderate/low penetrance genes, such as partner and localizer of BRCA2 (PALB2), checkpoint kinase 2 (CHEK2), ATM, DNA mismatch repair adenosine 5’-triphosphatase (ATPase) 1 (MSH1) or mutL homolog 2 (MLH2) [hereditary non-polyposis colo-rectal cancer (HNPCC) or Lynch syndrome], CHEK2, caspase 8 (CASP8), peripheral-type benzodiazepine receptor (PBRL), and BRCA1interacting protein 1 (BRIP1). Moderate-risk genes associated with syndromes that are inherited in an autosomal dominant pattern (such as Cowden disease, HNPCC, MuirTorre syndrome, and Peutz-Jeghers syndrome) exhibit lower penetrance and thus less risk of breast and/or ovarian cancer. Several other less frequently occurring predisposition genes such as Harvey rat sarcoma (HRAS) or the ER gene, the androgen receptor (AR) gene, and cytosine-adenine-guanine (CAG) repeats of AR, as well as reproductive and hormonal factors may also be involved and may therefore modify cancer risk, but to a lesser extent. Other cancer-related genes [including myelocytomatosis (myc), c-erbB2, tumor susceptibility gene 101 (TSG101), and Prophylactic Breast Surgery 125 mammary-derived growth inhibitor (MDGI)] are involved in breast carcinogenesis, but they do not give rise to familial breast cancer syndromes. Multi-gene testing, if used appropriately, is generally a more cost- and time-effective method than single-gene testing, and may increase the number of patients who can be offered personal surveillance, risk-reduction options, and testing of high-risk family members. Recent advances in molecular genetics testing have identified a number of susceptibility genes related to hereditary breast and/or ovarian cancers other than BRCA1 and BRCA2. The introduction of multi-gene testing for hereditary cancer has revolutionized the clinical management of high-risk patients and their families. Individuals with hereditary breast/ovarian cancer will benefit from genetic counseling/testing [588]. Thus, the hereditary breast and ovarian cancer (HBOC) syndrome includes genetic alterations of various susceptibility genes such as p53, ATM, PTEN or MSH2, MLH1, protein homolog mismatch repair system component (PMS)1, PMS2, MSH3 and MSH6, and BRCA1 and BRCA2. Germline mutations of the cancer-susceptibility genes BRCA1 and BRCA2 seem to be the major etiology of the HBOC. Male carriers have a moderately increased risk of prostate cancer. Genetic counseling and identification of high-risk families may be essential: 1) to provide the best method for genetic testing by explaining the sensitivity and specificity of the methods; 2) to offer the opportunity to participate in specific early cancer detection programs [breast (self) palpation, ultrasound, mammography, and MRI tomography for breast cancer, and vaginal exploration and ultrasound for ovarian cancer]; 3) to inform them about prophylactic medication (oral contraceptive pill), chemoprevention (TMX, raloxifen, and aromatase inhibitors) or surgery (bilateral prophylactic mastectomy or salpingo-oophorectomy); and 4) to provide individualized psychological support. To fulfill these broad demands, an interdisciplinary counseling approach (gynecological oncology, human genetics, molecular biology, and psychotherapy) in the setting of a cancer genetic clinic seems the most appropriate. There, participation in predictive genetic testing or the use of preventive or therapeutic options may be discussed extensively with the subjects. In particular, preventive options are emotionally disturbing for the subjects, and in cases of previous cancer. Breast cancer chemoprevention for high-risk women does not seem to be as effective as expected. However, oral contraceptive pill reduces the risk for ovarian cancer. For prophylactic surgery, various points have to be considered, including: 126 Ciro Comparetto and Franco Borruto 1) 2) 3) 4) individual risk assessment and gain in life expectancy; value of screening and early detection methods or medical prevention; disease characteristics and prognosis; and anxiety and quality of life. Decisions regarding these options have to be individualized and psychological support must be offered during the period of decision and follow-up [589]. The risk of breast and ovarian cancer by age 70 in a BRCA1 mutation carrier is estimated at 55-75% and 16-26%, respectively, overall, and as high as 87% and 44% in those with a strong family history. The cancer risks associated with BRCA2 mutations appear to be somewhat lower than those of BRCA1. BRCA mutations show a strong founder effect. This is best recognized in the Ashkenazi Jewish community, in which the incidence of one of three characteristic mutations is about 2%. In other ethnic groups, the pattern of mutations is different, with over 100 distinct mutations throughout the genes having been described. Most mutations so far have been frame-shift or mis-sense mutations, although large deletions have also been described. Thus, in most situations, assessment of the whole coding sequence is required to confirm or exclude a mutation. Microarray geneexpression studies show that breast tumors from BRCA1 carriers are predominantly of basal subtype (i.e., triple-negative) and BRCA2 carriers are of luminal subtype (i.e., ERpositive). Guidelines to suggest who is likely to be a mutation carrier are being clarified, but the appropriate management of someone who tests positive remains difficult. Prophylactic mastectomy and salpingo-oophorectomy are likely to offer substantial gains in life expectancy to mutation carriers, especially for young women with a strong family history. Unfortunately, there are no currently available strategies to eliminate the risk of breast or ovarian cancer. The psychological impact of testing also remains poorly understood, and the danger of various forms of discrimination remains too. These factors must be clearly understood by all parties prior to testing. The process of a dynamic, interactive informed consent – much more than a simple printed document – and also counseling is central to the testing process [590]. Yet, many women with a personal or family history suggestive of a hereditary susceptibility to breast cancer undergo genetic testing and no significant genetic alteration is found. Thus, there are other susceptibility genes that have not been identified, and it is likely that the remaining familial contribution to breast cancer will be explained by the presence of multiple low penetrance alleles that coexist to confer high penetrance risks (a polygenic model). The American Cancer Society (ACS) has identified cancer prevention as a key component of cancer management and there is interest in developing individualized cancer prevention focused on identifying high-risk individuals who are most likely to benefit from more aggressive risk-reduction measures. Breast cancer risk assessment and genetic counseling are currently provided by genetic counselors, oncology nurse specialists, geneticists, Prophylactic Breast Surgery 127 medical and surgical oncologists, gynecologists, and other health care professionals, often working within a multidisciplinary clinical setting. Current methods for risk assessment and predictive genetic testing have limitations and improvements in molecular testing and risk assessment tools is necessary to maximize individual breast cancer risk assessment and to fulfill the promise of cancer prevention [591]. Low-risk genes likely require significant environmental exposure, and although they are associated with the lowest risk of cancer, they account for more cancer than highand moderate-risk genes. Lifetime risks for breast or ovarian cancer can be estimated. The Gail and Claus models are the more widely utilized models for calculation of lifetime breast cancer risk. Models are also available for determining the likelihood of finding a BRCA1/2 mutation (the BRCAPRO and Myriad models). Appropriate candidates for testing include affected individuals (women, but occasionally men) who are most likely to have a hereditary form of cancer. Testing should proceed only after a thorough discussion of the risks, benefits, and limitations of testing. Risk-reducing options are available to women with a strong family history of breast and ovarian cancer. These options include high-risk screening, chemoprevention, and prophylactic surgery [592]. Women at particularly high risk of developing breast cancer represent a group in whom expensive and rigorous screening programs are cost-effective and who may benefit from trials of chemoprevention. There are only preliminary data on the efficacy of increased surveillance and on risk reduction due to prophylactic surgery. However, for chemoprevention to be equivalent to prophylactic mastectomy, it will be necessary to strive for an equivalent reduction. The efficacy of chemoprevention in this high-risk population is unknown. Existing and new agents for chemoprevention need to be carefully assessed in properly designed clinical trials among such women. In the process, other factors modifying the penetrance in mutation carriers need to be taken into account in order to evaluate the true effect of the chemopreventive agents. Polygenes confer much lower levels of risk and may be relevant for risk assessment when the effects of multiple loci, possibly in conjunction with environmental factors, are understood and quantified. At present, it seems unlikely that the genetic information at single polygenes will be clinically relevant for risk assessment and management [593]. Breast cancer occurs in hereditary and sporadic form. However, not all mutations will lead to breast cancer, and to prevent unnecessary surgery, assays to determine which mutations adversely affect the functions of the protein encoded by the BRCA genes are being developed. The functions of BRCA1 are mediated by numerous interactions that are required for cell-cycle and centrosome control, transcriptional regulation, and the DNA damage response. Mis-sense mutations that perturb the interactions of BRCA1 will adversely affect these functions and are, therefore, likely to lead to breast cancer [594]. BRCA1 mutations are rare in sporadic cancers, but loss of BRCA1 resulting from reduced expression or incorrect subcellular localization is postulated to be important in 128 Ciro Comparetto and Franco Borruto non-familial breast cancers. More than 300 germline mutations have been identified so far in patients with familial breast and/or ovarian cancer, however, only a few somatic mutations have been identified in sporadic breast cancer. The decreased expression of BRCA1 in sporadic breast cancer is thought to be caused by other mechanisms, such as 5’-cytosine-phosphate-guanine-3’ (CpG) methylation. BRCA1 expression may play an important role in the pathogenesis and prognosis of sporadic breast carcinoma [595]. The “cancer stem cell” (CSC) hypothesis proposes that tumors arise in stem or progenitor cells generating in tumors driven by a subcomponent that retains CSC properties. Recent evidence supports the hypothesis that the BRCA1 gene involved in hereditary breast cancer plays a role in breast stem cell (BSC) function. Furthermore, studies using mouse BRCA1 knockout models provide evidence for the existence of heterogeneous CSC populations in tumors generated in these mice. Although these populations may arise from different stem/progenitor cells, they share the expression of a common set of stem cell regulatory genes and show similar characteristics in in-vitro mammosphere assays and xenograft models. Furthermore, these CSC display resistance to chemotherapeutic agents. These studies suggest that breast tumors may display intertumor stem cell heterogeneity. Despite this heterogeneity, CSC may share common characteristics that can be used for their identification and for therapeutic targeting [596]. Basal-like breast cancers are characterized by their unique expression profile, with the frequent loss of BRCA1, caused by such mechanisms as promoter methylation and the overexpression of high-mobility group proteins of the A type 1 (HMGB1) or inhibitor of differentiation 4 (ID4). Clinical-pathologically, basal-like cancers are ER-, PR-, and HER-2-negative: they are of high grade and have a poor prognosis. The fundamental similarity between BRCA1-mutated and basal-like cancers indicates that disruption of BRCA1 may be an essential common initial pathogenic event. Furthermore, p53 mutation and EGFR overexpression occur similarly in BRCA1-mutated and basallike cancers: these shared alterations provide very important information for understanding not only the genetic and epigenetic carcinogenic pathways in these tumors but also therapeutic strategies. Despite the limited available clinical data about response to chemotherapy, anthracycline-based chemotherapy seems to be effective in a distinct subset of basal-like cancers. Both disrupted BRCA1 and overexpressed topoisomerase IIalpha (TOP2A) possibly found in basal-like cancers are speculated to be associated with their increased sensitivity to anthracyclines. If these tumors respond to this chemotherapy, a favorable prognosis might be expected: however, in patients who do not respond, the prognosis is poor. Currently, the sensitivity of basal-like cancers to taxanes is not clear, but considering that these tumors have disrupted mitotic checkpoint function, a poor response may be suggested. On the basis of in-vitro studies, BRCA1disrupted basal-like cancers may be sensitive to DNA-damaging agents including platinum-based compounds, TOP1 and TOP2 inhibitors, and alkylating agents. In future, Prophylactic Breast Surgery 129 new therapeutic approaches for patients with basal-like cancers that are unlikely to respond to chemotherapy should focus on molecules that are involved in the pathogenic pathways of this disease [597]. Genome-wide association studies have already identified novel risk alleles with a series of tumor-initiating single-nucleotide polymorphisms (SNP). Some of these variants and other novel SNP and copy-number variants (CNV) may also be relevant for local failures (ipsilateral breast cancer/contralateral breast cancer). Beyond established risk factors, genetic testing allows identification of high-risk patients (BRCA mutation carriers) who may benefit from bilateral mastectomy rather than breast-conserving surgery. Human genetic variation (SNP/CNV) and DNA methylation may be relevant for local failures assessment. Technological revolution has opened a new avenue but multiple challenges should be overcome to integrate SNP/CNV as markers for ipsilateral breast cancer/contralateral breast cancer risk-stratificationbased personalized surgery [598]. It has now been well documented that MRI of the breast has a higher sensitivity than mammography for the diagnosis of breast cancer in patients predisposed to breast cancer. A new diagnostic device, molecular breast imaging (MBI), is now available and may be as sensitive as MRI. To date, this exciting technology, MBI, has not been used in studies of patients with BRCA1/2 genes. Female BRCA1/2 mutation carriers face unique choices regarding management of their high risk for breast and ovarian cancer that impact their reproductive options. Despite their high levels of concern, few female BRCA1/2 mutation carriers consider assisted reproduction technologies (ART) such as pregnancy surrogate, cryopreservation of oocytes or embryos, or implantation genetic diagnosis to select embryos without BRCA1/2 mutation. Further research must be undertaken to explore the risk management of patients with inherited cancer predisposition and to incorporate these preferences into clinical care [599]. Risk estimation is the most important clinical implication. Despite inadequate knowledge about the genetic predisposition to breast cancer and its clinical implications, the demand for genetic testing is likely to expand rapidly. In addition to risk estimation, cancer surveillance, and preventive strategies, gene therapy offers a new and theoretically attractive approach to breast cancer management [600]. Because of the complex nature of genetic testing, mutation analysis is not presently routinely available outside genetic counseling clinics. The need to provide comprehensive counseling for women with an inherited predisposition to breast cancer has seen the evolution of the familial cancer clinic, involving a multidisciplinary specialist team approach. Familial cancer clinics will provide individuals with information about their risk of developing breast cancer and offer advice regarding further management strategies. It is important that surgeons, who have traditionally played a key role in breast cancer treatment, remain cognizant of these advances in genetic molecular biology, and in so doing continue to remain key participants in the conduct of breast cancer management [601]. 130 Ciro Comparetto and Franco Borruto The USA National Comprehensive Cancer Network (NCCN), the United Kingdom (UK) National Institute for Health and Care Excellence (NICE), and the ASCO have recently updated relevant guidelines that inform practice on breast cancer screening and prevention strategies for women at high risk of the disease. In the recently published literature, there have been several important findings. A meta-analysis of RCT of selective ER modulators (SERM) further support the use of SERM and aromatase inhibitors in the primary prevention of breast cancer. A large observational study has provided evidence that the SERM TMX may be efficacious for breast cancer prevention in women who carry mutations in the breast cancer predisposition genes, BRCA1 and BRCA2. Several observational studies have suggested that contralateral risk-reducing mastectomy, following a diagnosis of breast cancer, may reduce mortality [602]. The USA Preventive Services Task Force (USPSTF) recommends that primary care providers screen women who have family members with breast, ovarian, tubal, or peritoneal cancer with one of several screening tools designed to identify a family history that may be associated with an increased risk for potentially harmful mutations in breast cancer susceptibility genes (BRCA1 or BRCA2). Women with positive screening results should receive genetic counseling and, if indicated after counseling, BRCA testing. The USPSTF recommends against routine genetic counseling or BRCA testing for women whose family history is not associated with an increased risk for potentially harmful mutations in the BRCA1 or BRCA2 genes [603]. The concept of prophylactic mastectomy was nurtured in the shadow of the radical mastectomy. It evolved as preferable to the mutilation caused by the procedure. It developed during a time when the difference between benignancy and malignancy was not as clear and when patients with benign disease were thought to be at significant risk. The idea of surgical prophylaxis accompanied by a superior cosmetic result, in comparison to the radical mastectomy, is a noble one. In retrospect, however, it is clear that the indications were ill-defined, based often on unfounded risk, and predicated on patient and physician anxiety. The scope of risk in breast cancer has been narrowed, with new information identifying only specific subsets of women with proliferative types of benign disease as more susceptible to the subsequent development of carcinoma. Extensive reviews of material taken at biopsy that had been validated longitudinally have provided data to substantiate this contention. The concept of familial high risk must take into account the number of affected family members, age at diagnosis, menopausal status, and bilaterality. The majority of indicants that motivated and propitiated the performance of the bulk of prophylactic mastectomies have lost their relevance. Prophylactic mastectomy for carcinoma, therefore, can perhaps be reserved for women with biopsy-proved, high-risk lesions or an exceptional familial risk, or both, or hereditary risk. Such women must choose for themselves and accept the uncertainty of hypothetic risk reduction, life-long continued surveillance, and an altered body image. Prophylactic Breast Surgery 131 Guiding patients in the decision should involve a multidisciplinary team composed of a surgical oncologist, geneticist, pathologist, psychotherapist, and plastic surgeon. As a concept, the reduction of risk is appealing, but remains yet to prove itself superior to rigorous clinical surveillance with high-quality mammography. The experience reflected in the literature of a seemingly low rate of subsequent carcinoma cannot be judged, because it seems that operations were applied indiscriminately to patients selected by unknown means and from an unknown population pool. Success based on protecting those not at increased risk only invalidates the operation further. Most surgical and medical oncologists recognize that breast cancer is either localized or disseminated at the time of the initial diagnosis [604]. Bilateral prophylactic mastectomy is effective in reducing the risk of breast cancer in women with a well-defined family history or in women with BRCA 1 or 2 mutations. Evaluating patient-reported outcomes following bilateral prophylactic mastectomy are thus essential for evaluating success of bilateral prophylactic mastectomy from patient’s perspective. Postbilateral prophylactic mastectomy, patients are satisfied with the outcomes and report high psychosocial wellbeing and positive body image. Sexual well-being and somatosensory function are most negatively affected. Vulnerability, psychological distress, and preoperative cancer distress are significant negative predictors of quality of life and body image postbilateral prophylactic mastectomy. There is a paucity of high-quality data on outcomes of different health-related quality of life domains postbilateral prophylactic mastectomy. Future studies should strive to use validated and breast-specific instruments for measuring health-related quality of life. This will facilitate shared decision-making by enabling surgeons to provide evidence-based answers to women contemplating bilateral prophylactic mastectomy [605]. Mastectomy rates have significantly increased over the last decades, likely due to the rising trend of risk-reducing mastectomies in the treatment and prevention of breast cancer. Growing evidence suggests that aggressive risk-reducing surgical strategies are only justified in high-risk breast cancer situations. Notably, in this selected cohort of women, prophylactic mastectomies offer evident benefit for local and contralateral disease control, and may also provide a survival benefit. Nevertheless, the extent of the increasing frequency of this operation is not explained by the broadening of the medical indications alone [606]. The Society of Surgical Oncology (SSO) has developed a position statement that lists conditions warranting consideration of prophylactic mastectomy. It must be stressed that there are no absolute indications for prophylactic mastectomy. The data are limited about the efficacy of prophylactic mastectomy in humans, but recent studies suggest that it results in up to 90% reduction in the risk for breast cancer. Total mastectomy is technically a more definitive procedure, although reported series have had a predominance of patients undergoing subcutaneous, nipplesparing procedures. Prophylactic mastectomy may improve longevity in BRCA mutation 132 Ciro Comparetto and Franco Borruto carriers, but this must be balanced against the impact on quality of life. The benefits of prophylactic mastectomy relative to chemoprevention are unclear because there are no prospective RCT comparing these two strategies. Contralateral prophylactic mastectomy in patients with a unilateral cancer is unlikely to improve survival, but this approach may be considered for high-risk or difficult-to-observe patients, to facilitate breast reconstruction, and for the psychological benefits. Patients considering prophylactic mastectomy should be well informed of risk-reduction alternatives and the limitations in the efficacy and cosmetic results of the procedure [607]. The surgical technique should aim at removing substantially all at-risk breast tissue. However, there is an obvious balance between reduction of cancer risk and cosmetic outcome. The surgical technique involves several operations to include the risk-reducing mastectomy as well as breast reconstructive procedures. Skin-sparing mastectomy represents a new surgical approach that allows a mastectomy, whereas preserving the natural skin envelope of the breast. Breast reconstruction will involve several operations, especially if the NAC is resected and is subsequently reconstructed. The contraindications to risk-reducing mastectomy include the following: 1) the status of the family history or Munchausen’s syndrome has not been confirmed; 2) the risk-reducing mastectomy is not the women’s own choice; 3) the patient has a current psychiatric disorder including clinical depression, cancer phobia, or body dysmorphic syndrome; 4) if the comorbidity outweighs the clinical benefits, surgery should not be undertaken; and 5) finally, the patient must not have unrealistic expectations of the benefits of surgery: she must understand the subsequent risk-reducing mastectomy may significantly reduce, but not eliminate the risk of subsequent breast cancer [608]. Because the cosmetic result of immediate breast reconstruction may not be optimal, nipple sensation is lost, and capsule contraction may cause problems when silicon prostheses are used, the patients should be well informed of risks and other preventive methods. Surveillance or chemoprevention are suggested as alternatives to risk-reduction mastectomy, but there is lack of prospective RCT comparing these options. Because BRCA1 and BRCA2 gene mutations also increase risk of ovarian cancer, management of these patients should be shared by the breast surgeon and gynecologist [609]. For the majority of high-risk women, however, bilateral prophylactic mastectomy is not an acceptable option for primary prevention of breast cancer. Several epidemiological follow-up studies have indicated that there may be a substantial reduction in breast cancer risk among women who have undergone breast reduction surgery. Although such Prophylactic Breast Surgery 133 observational studies cannot demonstrate definitively that reduction mammoplasty reduces the risk of breast cancer, the evidence from these studies is sufficiently strong to warrant the evaluation of breast reduction surgery as an option for primary prevention in clinical studies of women at increased risk of breast cancer. The availability of a more acceptable surgical option for primary prevention of breast cancer could increase the number of women willing to choose risk-reduction surgery and thus may result in an overall reduction in breast cancer mortality among high-risk women [610]. First, a risky condition is not a disease and prevention does not improve well-being. The benefits are only statistical and make sense at the population level. Secondly, the cause of the risk is a genetic factor and some might argue about genetic “exceptionalism.” Thirdly, there is no organ as connected to femininity, sensuality, sexuality, adulthood, and motherhood as the breast. Lastly, making tough and complex choices requires assistance from ethics. Among ethical principles, western countries often rely on autonomy. The physician has to deliver all the relevant information: based on this “knowledge” and using their own values, patients will take a decision. In 1998, in France national recommendations set a list of criteria to fulfill, reducing autonomy. It might be expected that this tough issue will be solved, thanks to the improvement of prevention and therapeutic efficacy [611]. Finally, there must be a balance in the reduction in risk against cosmetic outcome, with subsequent quality of life issues. Women should be offered risk-reduction mastectomy only on the basis of a strict selection and management plan, like that used in Manchester protocol. This protocol involves a minimum of two sessions with a geneticist/oncologist, a session with a psychiatrist, and two sessions with a plastic and reconstructive surgeon with the support of a breast care nurse. A simple formula to calculate the life-time risk of contralateral breast cancer has been devised. This allows stratification of breast cancer patients into different risk groups: low, above average, moderate, and high risk. Recommendations vary according to different risk groups. These guidelines are a useful tool for clinicians counseling women requesting contralateral risk-reducing mastectomy. Risk assessment is mandatory in this group of patients, and this formula allows evidencebased recommendations to be made [612]. In conclusion, the new genetics is having an impact on many areas of healthcare. Diversity in the genetic code accounts for differences in phenotypes between populations and it is becoming apparent that genetic differences may have a role in predisposition to and behavior of disease. Genetic models suggest that there are two types of genetic predisposition to disease: the so-called high- and low-penetrance genes. At present, most of the impact on medicine has been from highly penetrant genes, and genetic testing for disease predisposition, particularly for diseases of late onset (e.g., certain cancers) is in its infancy. As a general statement, approximately 5-10% of common cancers are due to such highly penetrant genes. The category of genes that will become of increasing interest is that of the low-penetrance genes. Often, these are normal variations in genes 134 Ciro Comparetto and Franco Borruto that result in a slightly increased risk of disease. These are analogous to high blood pressure patients carrying an increased risk of cardiovascular disease. Once rapid genetic analysis is available for these types of genes, such analysis would be analogous to taking someone’s blood pressure by a general practitioner to identify individuals at increased risk of cardiovascular disease. This will produce a revolutionary change in the way we practice medicine. Genetic analysis will become faster and may therefore be more commonplace. It is possible to envisage an era when genetic analysis will become a routine part of primary care to identify changes in low penetrance genes that will confer a “risk profile” for patients. This will then enable their primary care physicians to advise about primary prevention and even prescribe certain preventive drugs to decrease the risk of certain diseases occurring. This proactive rather than reactive style of practicing medicine is potentially exciting, however it carries with it ethical, legal, and social implications for how we deal with this new knowledge [613]. The surgical option which should be reserved for patients with BRCA1/2 mutation and breast cancer diagnosis is still debated. Several aspects should be considered before the surgical decision-making: the risk of ipsilateral breast recurrence, the risk of contralateral breast cancer, the potential survival benefit of prophylactic mastectomy, and the possible risk factors that could either increase or decrease the risk for ipsilateral breast recurrence or contralateral breast cancer. Breast conservative treatment does not increase the risk for ipsilateral breast recurrence in BRCA mutation carriers compared to non-carriers in short term follow-up. However, an increased risk for ipsilateral breast recurrence in carriers was observed in studies with long follow-up. In spite of the increased risk for ipsilateral breast recurrence in patients who underwent breast conservative treatment than patients with mastectomy, no significant difference in breast-cancer specific or overall survival was observed by local treatment type at 15 years. Patients with BRCA mutation had a higher risk for contralateral breast cancer compared with non-carriers and BRCA1mutation carriers had an increased risk for contralateral breast cancer compared to BRCA2-mutation carriers. Bilateral mastectomy is intended to prevent contralateral breast cancer in BRCA mutation carriers, however, no difference in survival was found if a contralateral prophylactic mastectomy was performed or not. For higher-risk groups of BRCA mutated patients, a more aggressive surgical approach may be preferable, but there are some aspects that should be considered in the surgical decision-making process. The use of adjuvant chemotherapy and performing salpingo-oophorectomy are associated with a decreased risk for ipsilateral breast recurrence. When considering the risk for contralateral breast cancer, three risk factors were associated with significantly decreased risk: the use of adjuvant TMX, performing salpingo-oophorectomy, and older age at first breast cancer diagnosis. As a result, we can identify a group of patients that might benefit from a more aggressive surgical approach (unilateral mastectomy or unilateral therapeutic mastectomy with concomitant contralateral prophylactic Prophylactic Breast Surgery 135 mastectomy). For women with BRCA mutations candidate to mastectomy, preservation of the NAC may be highly important due to the generally younger age at time of surgery. Concerning the oncological safety, nipple-sparing mastectomy is an acceptable option, with no evidence of compromise to oncological safety at short-term follow-up. The evaluation of surgical treatment in breast cancer patients with BRCA 1/2 mutation should include several issues, namely the current evidence of adequate oncological safety of breast conservative treatment in BRCA-mutated patients, the increased risk for contralateral breast cancer especially in BRCA1 carriers, the feasibility on nipple-sparing mastectomy with a greater patient’s satisfaction for cosmetic results with no evidence of compromised oncological safety, and, finally, the awareness that breast radiotherapy might increase the risk of complications in a possible subsequent mastectomy with immediate breast reconstruction [614-622]. Chapter IX Breast Reconstruction Breast cancer is now treated either by conservative therapy or by mastectomy. In the first case, no reconstruction is usually necessary, although some patients require additional surgery for asymmetry, distortion, or even severe damage, by surgery or radiotherapy, of the treated breast. In these cases, reconstructive surgery should be performed very carefully, taking full account of the risk of operating in irradiated tissues. Minor procedures are usually adequate, but major surgery, reconstruction with abdominal flap, is sometimes the only solution to solve difficult postradiotherapy disasters. When a mastectomy is the choice of the patient and the surgeon, immediate reconstruction is now performed more often than before, as expansive prostheses are now available, allowing immediate implantation without endangering the skin flaps. In most cases of mastectomy, however, reconstruction has been performed as a secondary procedure, in two stages if possible (volume and symmetry after the first and areola after the second). Most of the reconstructions are done by simple implantation of a prosthesis. When local conditions require a flap, the latissimus dorsi musculocutaneous has been the best choice for years, but the lower rectus flap is now taking over, as it gives the advantage of reconstructing a breast with autologous tissue [623]. Breast reconstruction is not a new idea. Techniques have been developing since the late 1800s. Postmastectomy breast reconstruction now is an integral and valuable part of the management of patients with breast cancer. It offers hope and restitution for the patient while lessening the ablation and psychological trauma of mastectomy. Present surgical techniques and prostheses now permit any woman who has had a mastectomy to be reconstructed. Whether a woman should have breast reconstruction must be decided only after full discussion of prognosis and attainable goals with the woman herself. A variety of choices of procedure, timing, and extent of surgery are available [624]. 138 Ciro Comparetto and Franco Borruto Breast reconstruction after mastectomy for breast cancer has usually been performed as a delayed procedure, often not less than one year after completion of postoperative radiation therapy and cytotoxic chemotherapy. The reason for delaying the procedure has been the increased risk of wound complications after the adjuvant therapy. The reconstruction has been even further delayed for patients with increased risk of recurrence in order to avoid a reconstruction “in vain” for a patient, who will succumb to the disease within a couple of years after the reconstruction. Breast reconstruction can also be performed immediately in conjunction with the mastectomy. The rather slow acceptance of this procedure has mainly been for practical reasons. The mastectomies are usually performed by general surgeons. The operation has not been centralized to larger hospitals, and the patients usually want to have the operation performed as soon as possible. It is therefore often difficult to arrange a joint operation by the general and the plastic surgeons. In addition, suspicions have been raised that enlargement of the dissection area could spread cancer and increase the risk of local metastases. The same types of operation can be used for delayed and immediate breast reconstruction. There are basically two possible ways of creating a new breast mound. One entails transferring muscle, subcutaneous fat, and skin into the area of reconstruction in sufficient quantity to shape a breast mound. The other possibility is to shape the breast mound mainly by inserting a prosthesis, similar in consistency to breast tissue into the area of reconstruction. For practical reasons, the implant has to be covered by a sufficient amount of soft tissue, and for this purpose a number of pedicled flaps can be used. The implant most commonly used is made of silicone gel and covered by a membrane to prevent the gel from oozing into the surrounding tissues. A recent innovation is an implant with textured surface in order to diminish capsule formation around the prosthesis [625]. The techniques available today allow reconstruction of the breast in almost all the cases even in poor local conditions. In 60-70% of the cases, the reconstruction can be performed with an implant inserted behind the pectoralis muscle. Special implants called expanders are inflatable progressively in the postoperative course thanks to a reservoir located subcutaneously. They provide a progressive distention of the teguments and a more natural shape after substitution of the expander with a definitive implant. The symmetry is usually obtained thanks to a contralateral plastic surgery, which allows at the same time histological check-up of the glandular tissue of the opposite breast. The NAC is usually reconstructed in a second stage under local anesthesia, using local flaps for the nipple and a tattoo for the color of the areola. In 30% of the cases, especially after radiotherapy when a salvage mastectomy is required, a flap reconstruction is preferred. The autologous tissue reconstruction with the rectus myocutaneous flap gives excellent cosmetic results and the most natural shape for the breast, but it is a more demanding technique requiring a good experience. In some occasions, the reconstruction with the latissimus flap can also be autologous but usually Breast Reconstruction 139 requires the addition of prosthesis. As we have said, in most cases, the reconstruction can be performed immediately. The delayed reconstruction is usually preferred when the adjuvant chemotherapy should be delivered as soon as possible after the mastectomy. Complications of the reconstruction such as local necrosis or infections, leading to implant removal or revision of the flap could be detrimental to the patient in delaying the start of the chemotherapy. It is not recommended to reconstruct the breast immediately in case of locally advanced breast cancer. Partial breast reconstruction using plastic surgery procedures can also be performed in case of quadrantectomy in order to obtain a better cosmetic result. Local glandular flaps, as well as specific incisions according to the location of the tumor in the breast allow the reshaping of the breast even in case of large resection and, therefore, provide an opportunity to increase the number of conservative treatment indications, especially in case of CIS [626]. The advantages of breast reconstruction are well understood: it helps to eliminate many of the psychological burdens with which mastectomy patients must contend and allows patients to participate in a normal lifestyle. Immediate breast reconstruction eases much of the initial psychological trauma of mastectomy. For patients who require postoperative radiation, reconstruction is often far less complex if done immediately than if delayed, even though radiation increases the chances of capsular contracture. The knowledge that immediate breast reconstruction is available may reduce patient’s reluctance to seek medical advice when they find a breast lump [627]. Postmastectomy radiotherapy is well known in the plastic surgery community for having a negative impact on expander-implant-based immediate breast reconstruction, although recently some technical improvements allow better results. Very recent papers would suggest that there is no difference in postoperative complications in patients receiving postmastectomy radiotherapy using modern techniques. However, study results are often biased by small groups of patients and by heterogeneity of radiotherapy timing, different surgical techniques, and measured outcomes. Papers amenable to plastic surgeons highlighted the highest rate of undesired results, although with recent advances such as delayed-immediate reconstruction or protective lipofilling. Postmastectomy radiotherapy remains an undesired event when pursuing an implant-based breast reconstruction, although it does not represent an absolute contraindication. The higher rate of complications reported by plastic surgeons and not by other specialists can be explained with the greater attention to aesthetic details, such as capsular contractures. Technical strategies to prevent complications now allow better results, should be well known, and improved if possible in the future [628]. Neoadjuvant radio-chemotherapy and immediate reconstruction for breast cancer are still under debate. But there are recent abstracts and articles which show that neoadjuvant radio-chemotherapy is feasible and could improve the clinical outcome of breast cancer patients. It seems that the concept of immediate implant delayed autologous breast reconstruction could be a safe procedure that is at 140 Ciro Comparetto and Franco Borruto least equivalent to primary autologous reconstruction [629]. Due to increasing indications for postmastectomy radiotherapy and a growing demand for breast reconstruction or augmentation, increasing numbers of patients are currently being exposed to both these treatments. In view of the wide range of available techniques for breast reconstruction, either prosthetic or autologous, and their various sequencing in relation to radiotherapy, physicians can be faced with numerous clinical situations requiring comprehensive knowledge of the topic. The available data indicate the feasibility of such combinations, although at the expense of increased risk of complications and less satisfactory cosmesis. Of the two methods of breast reconstruction: using autologous tissue or prosthesis, the former seems to provide better cosmesis and a lower risk of complications in conjunction with radiotherapy. To minimize the risk of unfavorable outcome, the techniques and timing of both breast reconstruction and radiotherapy should be given meticulous attention [630]. A lack of consistent data is available about optimizing cosmetic outcomes, reducing potential treatment-related toxicities, and defining important prognostic factors for women undergoing postmastectomy radiation therapy following breast reconstruction. Autologous tissue reconstructions represent less than 20% of all breast reconstructions and include several techniques. A multitude of small studies have suggested that autologous tissue reconstruction is associated with improved cosmetic outcomes and similar rates of complications compared with expander/implant reconstructions. With regards to autologous tissue reconstructions, the addition of postmastectomy radiation therapy has been suggested but not definitively associated with a decrement in cosmetic outcome compared with patients not receiving radiation. Expander/implant-based reconstruction appears to be the most common form of breast reconstruction with large, prospective, and retrospective series demonstrating that 20-30% of patients may require some type of revision/replacement with long-term follow-up based on large series. Whereas postmastectomy radiation therapy and the addition of regional irradiation has been traditionally associated with increased complications and worse outcomes with expander/implant-based reconstruction, recent data suggest that no difference in perioperative complications exists in patients receiving postmastectomy radiation therapy using modern techniques [631-634]. While the surgical emphasis remains on a cure for the cancer, experience with breast reconstruction has not demonstrated any increased rate of cancer recurrence, even when reconstruction is performed immediately following tumor resection. Advances in surgical technique and biotechnology have made postmastectomy reconstruction possible. The development of silicone gel and saline-filled implants as well as tissue expanders has revolutionized breast reconstruction. The elucidation of musculocutaneous flaps now provides the surgeon with the ability to transfer adequate quantities of vascularized tissue to reconstruct the surgical defects. The advent of microsurgical techniques has Breast Reconstruction 141 provided an additional reconstructive option, with free tissue transfer allowing the plastic surgeon to move musculocutaneous flaps from remote or distant sites to reconstruct the defect. The option of having the reconstruction immediately following the mastectomy procedure is now available to the patient. When reviewing the anatomy of the breast region, the surgeon must consider the mammary gland, its vascular supply, and its lymphatic system [635]. Anatomists and surgeons have underestimated the importance of understanding the anatomical connective frame of the inframammary region. The submammary fold does not originate as a self-governing unit but depends on breast mould and on a fine superficial fascial system suspension. A reliable and fine correction of inframammary fold contour in breast reconstruction may only be achieved by an empirical surgical procedure that exclusively concerns the restoration of the superficial fascial system [636]. Many surgeons are under the impression that the blood supply is clearly defined in textbooks. Unfortunately, the majority of textbooks supply inadequate information and illustrations can be misleading in many instances. None of the textbooks describe a segmental pattern of blood supply when in actual fact a basic segmental pattern does exist. The reason for inadequate information is the perpetuation of facts since the work of the pioneers Cooper and Manchot from one textbook to another. A paucity of research studies thereafter and the fact that the results of some of these studies did not find their way into textbooks is another contributing factor. Researchers concurred on the main sources of blood supply: these are internal thoracic, lateral thoracic, anterior intercostal, and acromiothoracic (thoracoacromial) arteries. However, the different research studies showed considerable variation in the branches from the main sources to supply the NAC. Even though the locations of the main sources of blood supply are constant, partial or complete absence of branches from the main sources does occur and therefore the blood supply to the NAC is unpredictable. Cognizance of the basic segmental pattern and the variations resulting from embryological development will be helpful for the surgeon to use or adapt a technique to minimize the risk of nipple necrosis [637]. Breast reconstruction procedures can be categorized as implant reconstruction, local tissue with implant, autologous tissue, and free flaps. Implant reconstruction, immediate or delayed, has been the easiest and most fulfilling experience for the surgeon and the patient. Local tissue with implant and autologous tissue are usually available to those patients with anterior chest tissue deficiency or those who prefer autologous tissue without the fear of implant material. Free flap reconstruction is often selected when no other procedures are appropriate for the patient. Individual procedures must be familiar to the patient and the surgeon. Refinement and NAC reconstruction are an intimate part of breast reconstruction, but these are usually the decisions made by the patient that must be respected [638]. The state-of-the-art is such that the knowledge of its availability and success has significant effect on the willingness of women to seek earlier diagnosis and 142 Ciro Comparetto and Franco Borruto treatment. However, reconstruction is not for everyone. Some issues involved are financial, others have to do with cosmetic appearance, and still others involve the medical and psychological affects. Identifying and understanding the issues from the point of view of the patient is important in providing support for the patient with breast cancer [639]. The goal of breast reconstruction is to reconstruct breasts which meet the patient’s expectations both psychologically and aesthetically, while adhering to the principles of sound oncological management. Breast reconstruction is usually started around three to nine months after mastectomy. The simplest method of reconstruction uses tissue available after mastectomy and a silicone implant. Breast reconstruction has undergone a steady evolution since the introduction of the silicone gel prosthesis in the early 1960s. Current restrictions of the use of breast implants have increased the reliance on autologous tissue reconstruction. The improvement in the quality of breast reconstruction can be attributed in part to a refinement in mastectomy technique. There is an increasing emphasis on skin preservation, which makes it easier to match the remaining breast. Local recurrence after breast conservation generally necessitates a total mastectomy. Radiation fibrosis and endarteritis interfere with skin blood supply and impair wound healing. Reconstruction in this setting has a potential for increased operative morbidity [640]. In a large study, Lee and colleagues report that women receiving implants after mastectomies for early-stage breast cancer experience lower breast cancer mortality than women not receiving implants. Assessment of survival patterns among women receiving reconstructive implants is complex given unique patient characteristics, disease attributes, and treatment patterns. The interpretation of reduced mortality from breast cancer must be assessed in light of significantly reduced risks of death from most other causes. In contrast, patients receiving postmastectomy implants had elevated rates of suicide, consistent with findings among women with cosmetic implants. Additional welldesigned investigations are needed to clarify survival patterns among women receiving reconstructive implants [641-644]. Mesh use in surgical breast reconstruction is becoming increasingly common. However, there is still no consensus on whether synthetic matrices or biological matrices produce the best outcomes. Breast reconstruction with a synthetic matrix produces comparable aesthetic outcomes to a biological matrix, with lower costs and complication rates. The individual results for complication rates show that biological matrixes are associated with lower infection rates and slightly lower capsular contracture, but higher hematoma rates, and slightly higher rates of skin necrosis and explantation – although many had postoperative radiotherapy. The majority of the studies used biological matrices, and there are no RCT directly comparing the two types of meshes: definite conclusions cannot be drawn from the available evidence. A RCT comparing these outcomes in synthetic and biological matrix use is needed [645]. Prosthetic-based breast reconstruction commonly involves device Breast Reconstruction 143 placement in either a total submuscular pocket or a partial subpectoral position for just superior pole coverage, with various possible strategies for inferior pole coverage. Historically, the pectoralis major muscle is managed either by suturing the muscle to the inferior flap or with marionette sutures. Alternatively, the device is placed under total muscle/fascia coverage (under the pectoralis major, plus the serratus anterior and rectus abdominis muscles or fascia). For many plastic surgeons, acellular dermal matrix (ADM) is now used instead to function as a sling or “hammock” supporting the periprosthetic pocket and thus covering the inferior pole of the device, attached to the pectoralis major muscle above and to the inframammary fold below. In addition to its added soft-tissue support in the inferior pole, ADM may help to stabilize the pectoralis major muscle along its inferolateral margin, create a well-defined inframammary fold, provide the opportunity to significantly increase intraoperative fill volume of the tissue expander, and reduce the incidence or severity of significant or symptomatic capsular contracture, particularly in a patient whose breast has been treated with radiation. In addition to its indications in primary breast reconstruction, ADM has been increasingly used in secondary revision reconstruction cases. It can be used to buttress capsulorrhapy and capsulotomy sites and it can be used to replace periprosthetic capsule following capsulectomy. While clinical experience is accruing for these indications, ADM continues to be used in primary and secondary breast reconstruction [646]. Combining the use of tissue expanders and implants with autogenous tissue techniques of breast reconstruction (the “bioengineered breast” concept) takes maximal advantage of the benefits associated with each method. This approach provides an additional reconstructive option that is particularly useful in patients who are not ideal candidates for either method alone. Reconstruction may be performed in an immediate or delayed fashion, and the aesthetic results are excellent [647, 648]. There have been numerous advances in breast reconstruction techniques of the past decades. The disappearance of the radical mastectomy, along with the increased frequency of smaller tumor detection, have contributed significantly to these changes. Furthermore, reliable studies have shown that breast reconstruction does not interfere with extirpative surgery or delay postoperative adjuvant therapy if indicated. Studies such as these have led increasing numbers of women to elect immediate breast reconstruction as opposed to delaying that reconstruction for months or even years after the tumor extirpation. The advent of successful breast reconstruction using autogenous tissue provided the most radical change to reconstructive techniques over the past 40 years. The TRAM flap was the first of these techniques to be introduced, in 1982, and has rapidly assumed a position of prominence among those techniques chosen for breast reconstruction. The lateral transverse thigh flap (LTTF) and the buttock flap, while requiring microsurgical technique, are important alternatives for those patients who choose autogenous tissue breast reconstruction and should be presented to women during 144 Ciro Comparetto and Franco Borruto the discussion of alternatives for breast reconstruction. Implant technology has continued to improve with the introduction of the tissue expander, the most important addition in the past decades. Investigations are currently underway to provide a long-term tissue expander that does not have to be removed and replaced by a permanent implant. The ultimate end result would be to create a more normal breast shape without firmness, and the use of stacked or directional expanders may allow more freedom in creation of the new breast shape to conform to the opposite side. Finally, NAC reconstruction has improved significantly as the tissues of the breast mound itself are used for the new NAC, thereby avoiding the transfer of grafts from distant sites which do not generally maintain their size or projection over time [649, 650]. Breast reconstruction with autologous tissue achieves more natural results and a better simulation of a real breast than reconstruction based on prosthetic implants. Unlike implant-based reconstructions, which tend to develop capsular contractures, the results of autologous tissue reconstruction tend to improve with time. In the long run, the costs of autologous breast reconstruction with TRAM flaps are equal to or lower than those of reconstruction with tissue expansion and implants. Consequently, autologous tissue is preferable for most patients, provided they are suitable candidates for the surgery required by autologous reconstruction [651]. While autologous fat grafting provides a natural filler and seems easy to harvest, autologous fat grafting in breast surgery is still problematic especially due to the high resorption rate associated with megavolume transfer. Despite this pending issue, there is growing interest in this method, which is becoming more and more widespread, as can be seen by the recent increase in the number of clinical studies. Recent studies have concentrated on new techniques to improve fat viability and graft intake. However, all of these studies use different protocols at each step of the procedure. Furthermore, results may vary depending on the technique used for fat harvesting and processing [652, 653]. The optimal method for breast reconstruction should be safe, reliable, and accessible for every patient, and it should display little or no donor-site morbidity. After comparing mammary implants, it has been found that autogenous breast reconstruction can create a ptotic, soft, symmetrical breast mound. Detailed analysis of the vascular anatomy of the abdominal wall has extended the uses of the TRAM flap to major chest-wall reconstruction, both as a transposition flap and as a free-tissue transfer. Although the most direct pathway to the paraumbilical perforators that supply the large skin island of the flap is from the deep inferior epigastric artery, numerous collateral pathways exist from above that recruit blood from the intercostal vessels and the internal mammary artery, even if it has been ligated or used for myocardial revascularization. Awareness of these collateral pathways and care to preserve them whenever possible, combined with the willingness to supplement blood flow with a microvascular anastomosis of the deep inferior epigastric vessels, allows the surgeon to use the rectus abdominis flap and its Breast Reconstruction 145 variations in almost any major chest-wall reconstruction [654]. So, the pedicled TRAM flap is the most common method of autologous breast reconstruction. Common risk factors for complications from pedicled TRAM flap reconstruction include smoking, obesity, and postoperative radiotherapy. Patients with these risk factors are often candidates for a vascular delay procedure whose purpose is to enhance the blood flow within the TRAM flap. Despite advances in free flap breast reconstruction, pedicled TRAM flap breast reconstruction remains an excellent option for unilateral breast reconstructions. Unlike microsurgical breast reconstruction, the pedicled TRAM flap does not require sophisticated postoperative monitoring and can be performed efficiently in any hospital setting. Furthermore, with the addition of a vascular delay procedure, pedicled TRAM reconstructions can be safely performed even in traditionally “highrisk” patients [655]. Modern trends in breast reconstruction using the TRAM flap have promoted adequate blood supply to the flap while minimizing donor-site defects in the anterior abdominal wall. The pedicled TRAM flap remains one of the most frequently used flaps, but the indirect blood supply in this flap has required many modifications and refinements. Such modifications have included the bipedicled TRAM flap, the free TRAM flap, and the supercharged TRAM flap. To avoid donor-site morbidities, the muscle-sparing free TRAM, DIEP, and superficial inferior epigastric artery (SIEA) flap were introduced. The DIEP flap requires meticulous technique but offers proven reliability and a low rate of complications. As surgeons become more comfortable with harvesting DIEP flaps, the frequency of usage seems likely to increase. The latissimus dorsi musculocutaneous flap, gluteus maximus musculocutaneous flap, and others may be selected when these modifications of free TRAM flap are unavailable or unusable [656]. The transverse musculocutaneous gracilis free flap is a valuable choice for autologous tissue, unilateral or bilateral breast reconstruction. This procedure is an excellent and customized option for immediate or delayed breast reconstruction in patients with small to moderate size breasts. Few descriptions of flap dissection and breast mound shaping are available [657]. Latissimus dorsi miniflaps can be used to reconstruct central and upper quadrant resection defects, replacing the volume excised with autogenous tissue. Partial mastectomy, axillary dissection, flap harvest, and reconstruction of the resection defect is performed as a one-stage procedure through a single lateral incision. This oncoplastic approach allows extensive local excision during breast-conserving surgery without cosmetic penalties in a group of patients normally treated by mastectomy [658]. The latissimus dorsi musculocutaneous flap provides a readily available local source of well-vascularized muscle and fat that can be used in conjunction with tissue expanders and implants to reconstruct the breast after mastectomy in both an immediate and a delayed fashion. The procedure is straightforward, consistent, and well tolerated by patients. Donor-site morbidity is minimal and the aesthetic results can be outstanding. Use of this technique is 146 Ciro Comparetto and Franco Borruto recommended as a versatile and predictable method for breast reconstruction [659]. The gluteal region is an excellent donor site for autogenous breast reconstruction, particularly when the lower abdomen is not suitable. The superior gluteal perforator flap involves microsurgical transfer of skin and fat from the buttock without muscle sacrifice. Gluteal myocutaneous flaps have been described, but they have several problems, including a short vascular pedicle, exposure of the sciatic nerve, and recipient vessel size discrepancy needing vein grafts [660]. The substantial experience with the superior gluteal free flap for breast reconstruction indicates that this is a more difficult but important free flap for breast reconstruction. Compared with the standard TRAM free flap, it is a much less forgiving operation with many specific technical details. With careful attention to details on flap design, recipient vessel selection anastomsis, and flap insetting, the success rate and morbidity of the gluteal flap operation are highly acceptable. For patients unsuitable for the TRAM flap for autologous tissue reconstruction, this is a very valuable alternative. It can achieve some spectacular results in breast reconstruction in terms of volume, replacement, and projection – even in very thin patients [661]. Muscle-sparing autogenous breast reconstruction has enhanced the multidisciplinary care that is available to patients who have breast cancer. The Rubens free flap is another choice for autogenous tissue breast reconstruction. It uses the fatty area in the region generally described as the hip overlying or just above the iliac crest. It is based on the deep circumflex iliac artery and is indicated as a secondary choice in the presence of a previous abdominoplasty or TRAM flap breast reconstruction. Closure of the abdominal wall musculature along the iliac crest is the most exacting portion of this operation and is a key to its ultimate success [662]. The DIEP flap has proven reliability, a low complication rate, and is applicable to many clinical scenarios. Avoidance of muscle sacrifice in the abdomen ultimately translates into greater patient satisfaction. The increased demands, in terms of surgical expertise, are more than offset by decreased postoperative pain and decreased donor site morbidity. The methods that were used to innovate the DIEP flap have been applied to other donor sites and the available options for patients have been expanded [663]. The DIEP flap is becoming a widely practiced method of autologous breast reconstruction. Although it has been shown to be a safe and reliable technique with acceptable morbidity, disadvantages include a comparatively higher incidence of venous congestion and total flap loss compared with autologous reconstruction with a pedicled or free TRAM flap. Venous congestion is reported in up to one third of cases of breast reconstruction with a DIEP flap. If venous congestion is detected and addressed intraoperatively compared with postoperatively, outcomes are significantly improved. A wide variety of techniques have been introduced to augment venous drainage to treat congestion and prevent flap failure [664]. Breast Reconstruction 147 Perforator-based microsurgical reconstruction of the breast has steadily increased since the introduction of the technique in the 1990s. The use of perforator flaps has allowed for the transfer of large amounts of soft tissue with decreased morbidity. For breast reconstruction, the DIEP flap, the superior and inferior GAP flaps, and the transverse upper gracilis flap are all options. An alternative source uses posterior thigh soft tissue based on profunda artery perforators, termed the profunda artery perforator flap. Preoperative imaging helps identify posterior thigh perforators from the profunda femoris artery. These are marked, and an elliptical skin paddle, approximately 27 × 7 cm, is designed 1 cm inferior to the gluteal crease. Dissection proceeds in a suprafascial plane until nearing the perforator, at which point subfascial dissection is performed. The flap has a long pedicle (approximately 7 to 13 cm), which allows more options when performing anastomosis at the recipient site. The long elliptical shape of the flap allows coning of the tissue to form a more natural breast shape. The donor site is well tolerated and scars have been hidden within the gluteal crease. Long-term follow-up is needed to evaluate for possible fat necrosis of the transferred tissue. This is an excellent option when the abdomen is not available because of the long pedicle, muscle preservation, ability to cone the tissue, and hidden scar [665]. The procedure appears to offer less postoperative pain, lower abdominal morbidity, and better preservation of the rectus muscles than the more conventional flaps. However, the major disadvantage of these flaps is that they can be difficult to harvest, resulting in longer operative times. The challenges in flap dissection are a result of the variability in the vascular anatomy of the deep inferior epigastric artery and its perforating branches through the rectus muscle. The location, number, and caliber of the perforators and the intramuscular trajectory of the deep inferior epigastric artery branches vary greatly not only from individual to individual, but from one hemiabdomen to the other. The establishment of a presurgical map of the vessels on the abdomen facilitates surgical planning and may decrease operating room time, reduce intraoperative complications, and possibly improve outcomes. The available techniques for preoperative planning are the currently available imaging modalities of hand-held Doppler, color Doppler (duplex) ultrasound, CT angiography, and MRI angiography [666]. The vessel selected depends on multiple anatomical and surgical considerations, and the decision-making process can be exceptionally time-consuming, in part because of the wide variation that occurs in vascular anatomy. Preoperative imaging can greatly improve the efficiency of the selection process. Doppler ultrasonography is the most frequently used modality for vascular mapping, but the results are mixed because most perforating arteries have a diameter of less than 15 mm, the threshold for reliable visualization with ultrasonography. A CT angiographic evaluation performed with the use of specific postprocessing and display techniques may be more accurate for identifying the most suitable vessel. CT angiography provides valuable information that can help optimize 148 Ciro Comparetto and Franco Borruto surgical planning, decrease time spent in the operating room, and improve the outcome of breast reconstruction surgery [667]. The use of preoperative CT angiography reduces the operative time, postoperative flap-related complications, and donor-site morbidity compared with Doppler ultrasonography. Preoperative CT angiography has the potential to reduce operative cost and increase efficiency in the operating room. Thus, preoperative mapping by CT angiography should be strongly considered for abdominally-based free flap breast reconstruction [668, 669]. In summary, microsurgical tissue transfer involves the use of excess skin and fat (flaps) from a remote location to reconstruct the breast. Most often, tissues are transferred from the abdomen and buttocks. Less commonly, thigh flaps are used. These operations can provide durable, aesthetic reconstructions. In addition, advances in microsurgical techniques have improved operative success rates to the range of 99%. The selection of an appropriate flap for microsurgical breast reconstruction is multifactorial and is based on patient and oncological factors. These factors include patient comorbidities, body habitus/availability of donor tissues, cancer stage, and the need for postoperative adjuvant radiation therapy, as well as the risk of cancer in the contralateral breast. Appropriate choice of flap and surgical technique can minimize the risk of operative complications. Additionally, several large series have established that microsurgical breast reconstruction has no impact on survival, or loco-regional/distant recurrence rates [670, 671]. There is no longer any doubt that free flaps can achieve the best breast reconstruction. Proof has been the rapidly increasing popularity of the method [672]. In breast reconstruction with a free flap, the selection of suitable recipient vessels remains one of the most critical decisions for the surgeon. Most surgeons use one of the branches of the axillary vascular system, the thoracodorsal vessels. In recent years, free autogenous tissue transfer for breast reconstruction has become increasingly common. The free TRAM flap and the more recently described DIEP flap are currently the methods of choice for postmastectomy breast reconstruction. For patients who cannot have a TRAM flap, free flaps from other donor sites (superior gluteal flap, inferior gluteal flap, Rubens flap, and lateral transverse thigh flap) also have become important options [673, 674]. Current recipient vessels for microvascular breast reconstruction include the internal mammary and the thoracodorsal systems [675, 676]. The number one cause of death in American women is heart disease. Studies have clearly shown the superiority of internal mammary artery grafts for coronary revascularization over other conduits or intracoronary techniques. As we operate on older patient populations, the need for internal mammary artery use for coronary artery bypass grafting (CABG) after autologous breast reconstruction may arise more frequently. As the number of patients we operate on who may later require their internal mammary artery for CABG increases, so must our understanding of the implications of our selection of recipient vessels for free autologous breast reconstruction too [677]. Breast Reconstruction 149 New techniques for postsurgical breast reconstruction have been recently introduced. Over the last decade, autologous fat from subcutaneous tissue has been used by plastic surgeons for face volumization. It has also been used with growing frequency for aesthetic breast enhancement. Currently, experts in this field are becoming increasingly aware of the potential of fat for breast reconstruction. These new procedures include autologous fat grafting with or without the enrichment with autologous stromal vascular fraction (SVF), platelet-derived growth factors (PDGF), and insulin. Fat grafting is increasingly popular and is becoming a common practice in plastic surgery for postmastectomy breast reconstruction and aesthetic breast augmentation. However, the safety of fat transfers to postmastectomy defects has been called into question in recent debates due to the, albeit rare, observations of their possible influence on local cancer recurrence. The majority of concerned opinions are based on the safety of fat supplementation with additional amounts of adipose mesenchymal and stem cells. In experimental conditions, these cells were shown to create pathophysiological microenvironments and promote neoplastic transitions. This raises the question as to whether breast reconstruction with enriched fat is sufficiently safe to be performed without scientific justification and whether the present legal regulations are sufficient to guarantee patient safety in small facilities. In a number of circumstances, patients who have undergone treatment in profit-oriented offices, outside the system of oncological or surgical centers, might have been left without any long-term cancer surveillance. Valid opinions have also been expressed on the grounds of the ethical doubts concerning the advertising used by cosmetic practitioners who, on occasion, tend to overemphasize the putative benefits of stem cell applications despite the scant support in evidence-based medicine (EBM). Real progress in this field is possible only in scientific research that relies on bioethical evaluation, properly planned clinical trials, and the judgment of peers [678]. The reported improvement of fat graft viability with these techniques likely depends on the presence in the SVF of multipotent resident adipose-derived stem cells (ASC). The clinical advantage derives from the plasticity of ASC and their ability to generate new functional adipose tissue and vessels. However, there is an ongoing debate regarding the possible interplay between breast tumor cells and resident or transplanted ASC for their capacity to locally secrete growth factors. Thus, concerns over the oncological safety remains a controversial and hot topic among scientists and surgeons. Basic science and laboratory research repeatedly show a potentially dangerous effect of ASC on breast cancer cells. Clinical research, although limited, continually fails to show an increase in breast cancer recurrence after breast fat grafting, with the exception of one small study on a subset patient population with intraepithelial neoplasm of the breast [679]. Most of the data in the literature concerning ASC is derived from in-vitro models, whereas the knowledge of ASC behavior in-vivo remains scarce. Recent reports concerning SVF/ASC enrichment of fat graft did not describe any significant worsening 150 Ciro Comparetto and Franco Borruto of prognosis for patients undergoing those procedures. However, further studies and longer follow-ups are needed to specifically define technical procedures and to confirm the safety of procedures of SVF/ASC enrichment during postsurgical breast reconstruction [680-689]. As the demand for postmastectomy breast reconstruction has continued to rise, options for the implantable soft-tissue replacement products which enhance the aesthetic and reconstructive outcome of these procedures has grown as well. While the most common product used in an alloplastic breast reconstruction is an ADM derived from human sources, many other options are currently available, each offering their own unique properties and benefits [690]. The use of ADM in many plastic surgery procedures, including breast reconstruction, has increased dramatically in recent years. While expander/implant reconstruction can be performed successfully with standard techniques, the introduction of ADM has added a new tool with which to achieve lasting, predictable results [691]. ADM have generated interest for their possible applications in secondary revisions following prosthetic breast reconstruction. These materials can be effective in a variety of situations, including implant displacement, synmastia, capsular contracture, incisional support, and pocket conversion. ADM can also be placed in the setting of delayed breast reconstruction and to augment nipple projection. These biomaterials have demonstrated feasibility and success for many complex deformities. However, there is an associated learning curve that includes an understanding of proper technique and patient selection [692]. The use of ADM has allowed for single-stage immediate breast reconstruction after mastectomy at a significantly decreased cost compared with two-stage expander/implant reconstruction. The use of a pedicled autologous dermal flap in the same fashion as ADM in women with larger, ptotic breasts has also allowed for single-stage immediate breast reconstruction with similarly low complication rates and without the added procedural cost of using ADM. There have been no prior studies evaluating whether the added procedural cost for ADM is costeffective relative to using an autologous dermal flap in single-stage immediate breast reconstruction following mastectomy. Sensitivity analysis showed that ADM was not cost-effective when the complication rate for autologous dermal flaps was below 20%. ADM is not a cost-effective technology in patients who can have an autologous dermal flap in single-stage immediate breast reconstruction [693-697]. Breast reconstruction is a multi-stage process, leaving many patients overwhelmed by the surgeries they have had to endure by the time they reach the final stages. Patients, however, are encouraged to complete their new breast by undergoing NAC reconstruction. These last two procedures improve the aesthetic result by transforming the surgically created breast mount into a more natural-appearing breast. This final touch enhances body image, restoring the patient’s feeling of wholeness. These procedures are mostly done in the office under local or no anesthesia, depending on the sensation Breast Reconstruction 151 present in the reconstructed breast. Creation of the NAC is the final and important component of the breast reconstruction process [698]. Along with continuing progress in reconstructive surgery of the breast, numerous techniques of NAC reconstruction have been developed. Although many technical descriptions of NAC reconstruction exist in the medical literature, insufficient evidence-based data are present about the outcome. The impact of NAC reconstruction is well described in the literature. However, it is astonishing that the plastic surgical literature lacks evidence-based trials addressing this issue. Clearly, more evidence-based trials are necessary to ensure that recommendations for a particular technique are based on solid scientific data [699]. With time and experience, some methods have been discredited to historical significance only while others have evolved to widely accepted concepts used by surgeons all over the world, which in turn contributed new ideas and modifications. In addition to those favorite techniques, others are reserved as second-line alternatives in specific situations. The principle criterion for a pleasing NAC is symmetry regarding several parameters: color, texture, size, and projection [700]. The ideal reconstructed NAC provides sustained projection, the fewest complications, and high levels of patient satisfaction. A variety of materials are available for projection augmentation, including autologous, allogeneic, and synthetic materials. To date, there has been no systematic review to study the efficacy, projection, and complication rates of different materials used in NAC reconstruction. The results revealed heterogeneity in the type of material used within each category and inconsistent methodology used in outcomes assessment in NAC reconstruction. Overall, the quality of evidence is low. Synthetic materials have higher complication rates and allogeneic grafts have nipple projection comparable to that of autologous grafts. Further investigation with high-level evidence is necessary to determine the optimal material for NAC reconstruction [701-703]. Breast reconstruction procedures were not recommended in the past for those who planned for subsequent childbearing because of the transposition of portions of the abdominal wall during the procedure into the anatomical position of the breast, implying possible adverse effects over the contour of these manipulated areas during pregnancy and delivery. Uneventful pregnancy and delivery can be anticipated in breast cancer survivors who had undergone breast reconstruction via TRAM or its derivatives with minor negative effects on either the breast or the abdomen [704]. There are reports of patients having normal full-term pregnancies following pedicled TRAM flap breast reconstruction. These are individual cases and there is only limited published evidence to support the safety of pregnancy following surgery of this nature. Little is known about the effects of pregnancy after free tissue transfer, therefore it has been difficult to advise patients, in this situation, what the recommended course of action should be. We can conclude that TRAM flap breast reconstruction is not an obstacle to normal pregnancy and delivery. Patients need to be guided through the potential risks to their health if they 152 Ciro Comparetto and Franco Borruto should become pregnant, before reconstruction takes place. Obstetric and oncological specialists, as part of the multidisciplinary setting, need to be involved early in pregnancy to further inform and counsel these patients [705]. Breast reconstruction has been shown to have a positive effect on the psychological well being of women with breast cancer. Numerous studies have demonstrated that reconstruction performed concurrently with mastectomy is oncologically safe. Recent refinements in surgical techniques and prosthetic technologies, development of novel tissue substitutes, and increasing use of adjuvant radiotherapy have led to changes in the practice of breast reconstruction following mastectomy. Nevertheless, although increasing numbers of women are choosing to undergo postmastectomy reconstruction, this trend is inconsistent across demographic subgroups. In addition, the paradigm of performing immediate reconstruction on allcomers is being challenged by increasing use of postoperative radiotherapy. It is now appreciated that the implications of performing reconstruction in the setting of radiotherapy are both profound and controversial. Finally, questions are being raised about the factors that influence the ultimate surgical goal, namely patient satisfaction. Mastectomy is often associated with significant psychological sequelae including distorted body image and sexual dysfunction. Breast restoration is assumed to allow a full emotional and physical recovery from a breast cancer crisis [706, 707]. The goal of postmastectomy breast reconstruction is to restore a woman’s body image and to satisfy her personal expectations regarding the results of surgery. Studies in other surgical areas have shown that unrecognized or unfulfilled expectations may predict dissatisfaction more strongly than even the technical success of the surgery. Patient expectations play an especially critical role in elective procedures, such as cancer reconstruction, where the patient’s primary motivation is improved health-related quality of life. In breast reconstruction, assessment of patient expectations is therefore vital to optimal patient care. Systematic measurement and management of patient expectations may improve patient education, shared medical decision-making, and patient perception of outcomes [708]. It is anticipated that investigations using newly developed, patient-reported outcome measures provide important information about outcomes following reconstruction, which in turn will facilitate the decision-making process for both patients and surgeons [709]. Achieving an aesthetic outcome following postmastectomy breast reconstruction is both an important goal for the patient and plastic surgeon. However, there is currently an absence of a widely accepted, standardized, and validated professional aesthetic assessment scale following postmastectomy breast reconstruction. Of the 12 different professional aesthetic assessment scales that exist in the literature, the most commonly used scale was the four-point professional aesthetic assessment scale. The highest score on the modified Scientific Advisory Committee’s (SAC) Medical Outcomes Trust (MOT) criteria was assigned to the ten-point professional aesthetic assessment scale. Breast Reconstruction 153 However, this scale has limited clinical usefulness due to its poor responsiveness to change, lack of interpretability, and wide range of intra- and inter-rater. A “gold standard” professional aesthetic assessment scale needs to be developed to enhance the comparability of breast reconstruction results across techniques, surgeons, and studies to aid with the selection of procedures that produce the best aesthetic results from both the perspectives of the surgeon and patients [710]. Patient-reported outcomes provide an invaluable tool in the assessment of outcomes in plastic surgery. Traditionally, patientreported outcomes have consisted of either generic or ad hoc measures. However, more recently there has been interest in formally constructed and validated questionnaires that are specifically designed for a particular patient population. To date, the most frequently used measures are still generic measures. The 36-Item Short-Form Health Survey (SF36) was the most frequently used and most successfully applied showing evidence of responsiveness in multiple settings. Other measures such as the Hospital Anxiety and Depression Scale (HADS), the Hopwood Body Image Scale (BIS), and the Rosenberg Self-Esteem Scale (RSE) were able to show responsiveness in certain settings but lacked evidence as universal tools for the assessment of outcomes in reconstructive breast surgery. Despite the recent advent of measures designed specifically to assess patientreported outcomes in the breast reconstruction population, there still appears to be a role for the use of generic instruments. Many of these tools would benefit from undergoing formal validation in the breast reconstruction population [711]. All studies reported good levels of satisfaction. Methodological deficiencies (small sample sizes, context and study designs, satisfaction assessment, and basic statistical analysis) limit the generalizability of the findings. Overall, studies suggested that patients were satisfied with breast reconstruction whatever the technique used, whereas age or procedure timing did not affect general satisfaction. Breast symmetry, size, shape, and scars were reported as influencing the patients’ score. NAC reconstruction positively influenced satisfaction; radiation before/after reconstruction achieved satisfactory cosmesis, and complications predicted dissatisfaction [712-716]. Follow-up of patients with breast cancer is directed to the early detection of recurrent or metastatic disease and the detection of new primary breast cancer. The survival benefit of early detection is limited to some patients with local failure or new primary tumors. That imaging is not used in follow-up of patients who have had breast cancer reconstruction is related to possible interference with this putative benefit by the reconstructive procedure. Such follow-up is accomplished by the patient’s own surveillance, clinical examination, and laboratory testing supplemented by imaging studies. Clinical follow-up trials of women who have undergone breast reconstructive surgery show no evidence that locally recurrent breast carcinoma is masked when compared with follow-up of women who did not undergo reconstructive procedures. Reshaping of the contralateral breast to match the reconstructed breast introduces the 154 Ciro Comparetto and Franco Borruto possibility of interference with palpation as well as mammographic distortion in some women. This is an uncommon practical problem except when complicated by fat necrosis [717]. Therefore, the data on surveillance imaging of the reconstructed breast are extremely limited. However, by assessing the potential role for imaging in this setting and applying the principles of screening, there is a potential theoretic advantage of surveillance imaging in a very small subset of women: those with autologous tissue reconstructions and moderate to high risk of recurrence. A prospective registry study of surveillance imaging in this target population would be the appropriate way to determine its benefit and its impact on survival outcomes [718-720]. In conclusion, remarkable advances have been made in the field of breast reconstruction, especially since general surgeons, patients, and the community as a whole have become more knowledgeable and accepting. This has provided an impetus for plastic surgeons to develop better techniques. In general, less time now elapses between ablative and reconstructive surgery, and frequently reconstruction follows immediately. Procedures requiring fewer steps with less donor and recipient site morbidity are favored for both the mound and NAC reconstruction. There is a definite trend toward submuscular implantation to minimize the negative effects of capsular contracture. With a deficiency of skin or muscle, the trend is toward using musculocutaneous flaps primarily, latissimus dorsi, and the rectus abdominis flap. Occasionally, microvascular flap reconstruction is indicated for extensive chest wall defects, e.g., postirradiation injury. Postoperative closure techniques in breast reconstruction have remained largely unchanged over the past 75 years, despite recent use of adhesives and subcuticular staples and the advent of self-anchoring barbed sutures [721, 722]. Reconstructed breasts are not capable of nourishment, frequently are not erogenous, and most often are not as pleasing to the eye as the contralateral breast. Yet, reconstruction in the majority of cases has improved the quality of life for those women who have developed breast cancer and offered hope to those women in a high-risk category for developing breast cancer. To those ends, the search for the perfect breast reconstruction will continue [723]. Advances in materials and techniques, especially those involving transposition of muscle and skin flaps, have made breast reconstruction possible for most women who undergo mastectomy for breast cancer. The availability of this option can alleviate the breast and chest wall deformity that results from virtually all local treatment of breast cancer. It is essential that the reconstruction surgeon be part of the breast cancer management team from the beginning of treatment planning and that this surgeon work closely with the general surgeon, medical oncologist, and radiation therapist as well as the adjunctive treatment team members. The patient’s clinical status and the type of local treatment will be significant determinants of the reconstructive options. For women with Stage I breast cancer, these decisions may be based largely on the oncologist’s local and adjunctive therapy procedures and the woman’s desire to Breast Reconstruction 155 proceed or delay. For women with systemic disease, all members of the breast management team may need to agree on the advisability and timing of reconstruction. For appropriate patients, who are aware of and accept the risks involved, contralateral prophylactic mastectomy and bilateral reconstruction can provide a good solution to an otherwise difficult problem. Bilateral reconstruction can produce exceptionally good aesthetic results and, because good symmetry is achieved in the initial surgery, it only costs about 5% more than unilateral reconstruction [724]. Central to all of the numerous decisions regarding the timing, type, and extent of breast reconstruction is the primary goal of the entire team: the best possible management of the breast cancer itself. The promise of attractive, symmetric, and natural appearing breasts, complete with a symmetric NAC, has eased somewhat the diminishment of self-esteem and the threat to femininity that can accompany the loss of a breast. By lowering fear, the widely recognized availability of breast reconstruction may encourage more women to monitor their breasts and seek diagnosis of changes and may influence selection of the type of local treatment if cancer is detected. Because of the psychological and cultural significance of the breast, the reconstructive surgeon must be particularly sensitive to the psychological and aesthetic expectations of the patient. Even in those patients with metastases and limited life expectancy, breast reconstruction can enhance the quality of life [725]. Continued close cooperation between the general surgeon and the plastic surgeon will assist the patient in her return as a functioning member of society and not mere survival as a mastectomy cripple [726-737]. Chapter X Aesthetic Breast Surgery Health is multidimensional. Mortality, morbidity, and cost are traditional health indicators, whereas outcomes research relates to quality of life and health-related quality of life [738]. Elective aesthetic cosmetic surgery of the female breast appears not to warrant the same attention to informed consent or authorization as other surgical procedures. Arguments for benefits do not need to be persuasive since the prospective patient is usually “sold on the idea.” More rather than less information is necessary where the surgery will not achieve what is claimed for it and where there are significant risks of harm. Augmentation mammoplasty does not provide a solution to problems of body image, self-esteem, and sexuality: it may result in considerable pain, suffering, financial and emotional cost, interference with life choices, and a loss of trust in health care professionals. In view of the emerging data which points to the doubtful efficacy and safety of the procedure and the prosthetic devices, and since elective cosmetic surgery is said to be a growth industry, checks and balances need to be in place to ensure women are informed participants in decision-making. They require the best possible advice, care, and support. Changing fashions and popular demand are forcing us to adapt higher standards in breast surgery [739]. There are many methods of measuring the breast and their clinical applications are well described in the literature. However, there has been no attempt to compare these various methods to allow the user to have a broad overview of the subject. All of the methods can be classified into those that measure: 1) volume; 2) shape; and 3) surface area. 158 Ciro Comparetto and Franco Borruto Each category consists of several methods that work through different mechanisms and they vary in their reliability and feasibility. Based on their mechanism, the volume measurement methods were further grouped into the natural shape methods, the stereological method, the geometrical methods, and the mathematical modeling method. More objective breast evaluation can be achieved if all three dimensions (volume, shape, and surface area) are considered. In the volume measurements, 3-D modeling and the MRI are the most reliable tools. Linear measurement (geometry) and mathematical modeling are less accurate but are more economical. In the shape measurements, besides the traditional linear measurement, 3-D methods that can deliver color-coded maps and Swanson’s 2-D photographic measurement system are capable of depicting and tracking breast shape changes after surgery. Although the surface area metric has not been used extensively, it has potential in clinical and research applications [740]. The first two dimensions constitute the breast footprint. The third dimension is the shape of the breast on the footprint. It is important for the surgeon to understand how change in each of the parameters can be affected. The upper and lateral breast borders are relatively mobile, but the inferior and medial breast borders are relatively fixed. All four borders can be changed with certain surgical maneuvers. The breast is a skin structure that is held in place by skin/fascial zones of adherence, and the breast itself is mobile over the pectoralis fascia. Measurements before and after breast augmentation, breast reduction, mastopexy, and mastopexy-augmentation have been obtained so that the surgeon can better predict results. The change in suprasternal notch-to-nipple distance and the change in suprasternal notch-to-inframammary fold distance have been measured. Being able to explain the issues and the potential changes makes it easier for a surgeon to manage patients’ expectations [741]. Breast symmetry, size, and shape are key components of aesthetic outcomes of augmentation mammoplasty, reduction, and reconstruction. Many have claimed that the 3-D scanning technique, which measures breast volumes directly and assesses the asymmetry of the chest and breast on a 3-D model, is superior to anthropometric measuring in accuracy, precision, and reproducibility. The documented methods of 3-D body surface imaging include laser scanning, stereo photography, and so on. To achieve ideal aesthetic results, individualized surgery planning based on a reliable virtual model of the prospective surgery outcome could be of considerable value in decision making and assisting in guidance for the surgery procedure. Additionally, the 3D scanning technique is applicable in postoperative monitoring of morphological change, notably, in a dynamic way. Another distinguishing feature is that it enables virtual division of breast volume, thus surgeons could virtually divide the breast volumes into portions using 3-D scanning during the programming and evaluation of surgery plans. However, because 3-D surface scanning cannot look through the breast substances and reach the interspace between the chest and posterior border of the breast/dorsal limit of the breast, the inframammary fold in larger breasts cannot be correctly imaged, leaving Aesthetic Breast Surgery 159 the preoperative inframammary fold reference lacking. Therefore, 3-D scanning is thought to be inaccurate in large and/or ptotic breasts. Another fact that prevents 3-D scanning from wide application is its high cost and lack of access [742]. Creating or recreating an aesthetically pleasing breast shape in reconstructive and aesthetic breast surgery is an act that most experienced “breast” surgeons will find self-evident. A simple three-step philosophical and hands-on approach will make it easier for young and unexperienced plastic surgeons to not only analyze the problematic breast but also come up with an easy surgical strategy to create reproducible results [743, 744]. Knowledge on the forces acting on a woman’s breast and on the mechanical properties of the breast tissues is important for studying the effects of plastic surgery techniques for breast reconstruction as well as for the design of cosmetic breast implants. Surprisingly, there are no data in the literature regarding mechanical loads on the breast tissues during daily or sport activities, and there are no coherent sources of data in regard to mechanical properties of the breast tissues [745]. Approximately 500,000 cosmetic breast operations are done every year. The majority of these are for cosmetic reasons and include breast augmentations, mastopexies, and breast reductions. Common procedures include breast reconstruction following mastectomy for cancer. Augmentations are most commonly performed using saline implants in strictly cosmetic situations and occasionally silicone implants in select cases. Complications are unusual, breast-feeding is usually possible, and mammographic cancer detection is accurate provided that specific mammographic views are obtained. Most cosmetic breast surgery is not covered by insurance with the exception of breast reduction [746]. Insurance companies evaluate the medical necessity for breast reduction surgery based on internal company medical policies, but the correlation of insurance company criteria to the scientifically established indications for reduction mammaplasty has never been studied. Insurance company medical policy requirements with respect to reduction mammaplasty are, in many cases, arbitrary and without scientific basis. Requirements for a specific volume of reduction, a minimum age, a maximum body weight, and a trial of conservative therapy are required by the majority of managed care medical policies, even though scientific support for any of these requirements is not evident in the medical literature [747]. Appropriate follow-up care, including early postoperative serial mammography, is essential for women receiving cosmetic breast surgery. Postoperative complications include infection, poor wound healing, and pain. Hypertrophic scar formation, which may disrupt the normal breast contour, may be reduced by gentle and frequent massage of the scar. Avoiding sun exposure and using sun screens may prevent hyperpigmentation of the scar. Reduction mammoplasty is associated with sensory loss, which is occasionally permanent. Special complications associated with augmentation mammoplasty include diffusion of silicone through the implant and unfavorable breast appearance secondary to capsular contracture. Capsular 160 Ciro Comparetto and Franco Borruto contracture may be prevented by regular implant exercises. Preoperative and early postoperative serial mammograms are essential to differentiate surgical from malignant changes [748]. Women seek reduction mammoplasty for alleviation of their symptoms of mammary hypertrophy, which may include upper back and neck pain, strain, or muscle spasm. Brassiere strap grooves develop mostly on the shoulders of women with hypertrophied breasts. Varying degrees of the deformity are observed in such cases. To date, there is no universally accepted definition and classification for brassiere strap groove deformity [749]. Many of these women also desire an improvement in their appearance. Augmentation mammoplasty is sought by women for enlargement of the breasts and to attain a well-proportioned figure. Women who have experienced postpartum involution of the breasts request augmentation mammoplasty to regain the size and shape of their breasts prior to pregnancy [750]. In the last decade, entirely new techniques in breast reduction and reconstruction have evolved. What remains unclear is whether or not these new procedures, some of which carry additional surgical risk and cost, are superior from a patient perspective. Thus, plastic surgeons are ever more reliant on clinical research to provide high-level evidence to facilitate clinical decision-making, as well as policy negotiations and advocacy [751]. Breast deformities and mastopexy continue to challenge plastic surgeons. Deformities such as Poland syndrome, tuberous breast, gynecomastia, and other congenital conditions are uncommon, therefore, management experience is often limited. Various techniques have been described, with no general consensus regarding optimal management. Mastopexy has become more common and is performed both with and without augmentation mammaplasty. However, a variety of techniques are available, and a thorough understanding of the indications, patient selection criteria, and techniques is important to optimize outcomes [752]. Degrees I and II ptosis and atrophy of the female breast can definitively be corrected by the modified periwinkle shell operation. More problematic are the long-term results after correction of a degree III condition, especially when silastic prostheses for augmentation have been used. The long-term results can be optimized by a combination of dermal and glandular mastopexy and mammary implants [753]. Mastopexy includes multiple skin incision design and parenchymal manipulation options. Patient evaluation includes assessment of goals, degree of ptosis, tissue volume, skin quality, and breast position on the chest wall. There are critical technical details for each of the incision options, the various methods of parenchymal manipulation, and implant placement. The potential for complications is greatest for combined augmentation and mastopexy. Although they are effective, mastopexy procedures have the greatest incidence of litigation among aesthetic breast procedures [754]. Currently, four techniques of short scar mastopexy have been described: the periareolar, the vertical, the inverted-T, and the L-shaped scar. A large number of techniques have been published for minimal ptosis, whereas for significant ptosis, the number of surgical Aesthetic Breast Surgery 161 options is limited. It is evident that limited scar techniques can be applied to all grades of ptosis, but there is no one technique that can satisfactorily correct all degrees of ptosis. Plastic surgeons should weigh the advantages and limitations of each technique to correctly address breast ptosis [755]. Mammoplasty performed with an inverted-T skin resection pattern is a useful technique to treat moderately or severely ptotic breasts. This method of skin resection is conducted via three incisional components: periareolar, vertical, and horizontal. A simple modified winch suture can be inserted with the inverted-T technique to reduce the length of the horizontal incision [756]. The objective of breast reduction surgery is to reposition the nipple, remove excess parenchyma, and tailor the skin to fit the new shape [757]. The four geometric differences between the normal and enlarged breast are: vertical excess, broadened base, horizontal excess, and a descended NAC. All breast reduction techniques use a specific pedicle and a separate skin incision pattern, so they should be named after the scar and pedicle used. Technique selection must consider the degree of hypertrophy and ptosis, the skin and gland quality, the patient’s requirements, and the surgeon’s experience and preferences. The comprehension of basic breast geometry, a universal language for communication and a simple algorithm to approach the breast reduction patient are valuable tools, particularly for the surgeon who is becoming acquainted with reduction mammoplasty procedures [758]. The evidence indicates that resection volume is not correlated directly to the degree of postoperative symptom relief. Increased breast resection weight may increase the risks of complication. The evidence is inconclusive on whether increased body mass index (BMI) is associated with increased risk of complications. Perioperative antibiotics may reduce the risk of infection associated with reduction mammoplasty, and in standard reduction mammoplasty procedures without liposuction, the use of drains is not beneficial. Reduction mammoplasty has been shown to improve quality of life [759]. Breast reduction is confirmed to be a very useful operation, being a cosmetic as well as symptomatically beneficial procedure. It is seen to be a cause of a major increase in the unit’s workload as its popularity increases [760]. Although plastic surgeons have empirically “known” of the benefits of reduction mammoplasty for their patients, a paucity of outcome studies have been reported. A systematic evaluation of patient-focused outcome measures demonstrated that consistent improvement in physical symptoms was found across most studies, as was a high degree of patient satisfaction (78-95% very or moderately satisfied), and some have shown improvement in body image and psychological well-being. However, although consistent improvements in patient quality of life have been identified after reduction mammoplasty, inconsistencies among study designs do not allow formal meta-analysis [761]. The goal in breast reduction surgery is to reduce volume but at the same time to maintain circulation, sensation, and breast-feeding potential [762]. Any pedicle can be 162 Ciro Comparetto and Franco Borruto used to achieve a good breast reduction. We need to look at the skin pattern as separate from the pedicle design. There are numerous (and good) combinations available. Different situations will determine which combination is preferable. A standard inferior pedicle Wise pattern technique has been performed and now variations of the vertical technique have been used. A dermoglandular or central pedicle is the most likely to achieve all three. This medial pedicle gives the best breast reduction results. On the other hand, a lateral pedicle is used for mastopexies so that the inferior and lateral tissue can be rotated up under the areola. When faced with a re-reduction of a previous inferior pedicle, the vertical technique can still be used, and an adaptation of the inferior pedicle can be very acceptable. The superior pedicle is reserved mainly for very small reductions or mastopexies, but still the medial pedicle allows better lateral resection even in small reductions, and the lateral pedicle with recruitment of tissue allows for some autoaugmentation in mastopexies [763]. The recent findings of the experience with the B technique have been quite helpful and rewarding. Drawing a lateral angle when planning the procedure obtains a more circular areola contour and avoids last moment modifications. Also, upper pole undermining allows for an upper pole horizontal plication in the cases of a simple mastopexy. These modifications have been used over the last 30 years with no special complications and very satisfactory results [764]. In all cases of moderate- to high-grade hypertrophy of the breast, with or without ptosis, the reduction mammoplasty with L-shaped suture lines proves to be an effective method that avoids unacceptable scars extending to the midline of the thorax, especially in the young patient [765]. The use of dermoglandular flaps in reduction mastopexy was advocated by Paul Tessier, who never published his method before his death in June 2008. Dr. Tessier is acknowledged as the “father” of craniofacial surgery, but he had interest in aesthetic surgery, and was quite proud of the technique he had developed using dermoglandular flaps in reduction mammoplasty. He had literally hundreds of techniques and methods that he had developed but which never found their way into print, both because of his enormous surgical schedule, and perhaps his self-imposed standards for anything that he published, which were almost impossibly high. The technique proposed by Dr. Gargano is similar in some ways to Dr. Tessier [766]. The standard split-thickness superior pedicle vertical mammoplasty technique sometimes suffers from tension on the NAC. A bisected full-thickness superiorly based flap offers two vectors for transposition within the context of superior pedicle vertical mammoplasty. The procedure increases both upper-pole fullness and projection of the breast while decreasing tension on the NAC. The surgical procedure contributes to a natural appearance of the breast. It should provide a useful and simple surgical option, increasing the versatility of the superior pedicle vertical mammoplasty technique [767]. A controversy exists between vertical mammoplasty and the “traditional” keyhole/inferior pedicle method of breast reduction. The breast can be considered a cone. Aesthetic Breast Surgery 163 Breast projection then is the ratio between the nipple projection and the breast base. Two key concepts need to be considered: the orientation of the ellipses during excision of breast tissue in breast reduction and the role of the breast base/inframammary fold. Breast projection is not determined by the scars [768]. Vertical scar mammoplasty, first described by Lötsch in 1923 and Dartigues in 1924 for mastopexy, was extended later to breast reduction by Arié in 1957. It was otherwise lost to surgical history until Lassus began experimenting with it in 1964. It then was extended by Marchac and de Olarte, finally to be popularized by Lejour. Despite initial skepticism, vertical reduction mammoplasty is becoming increasingly popular in recent years because it best incorporates the two concepts of minimal scarring and a satisfactory breast shape. At the moment, vertical scar techniques seem to be more popular in Europe than in the USA. A recent survey, however, has demonstrated that even in the USA, it has surpassed the rate of inverted T-scar breast reductions. The technique, however, is not without major drawbacks, such as long vertical scars extending below the inframammary crease and excessive skin gathering and “dog-ear” at the lower end of the scar that may require long periods for resolution, causing extreme distress to patients and surgeons alike. Efforts are being made to minimize these complications and make the procedure more user-friendly either by modifying it or by replacing it with an alternative that retains the same advantages. Although conceptually opposed to the standard vertical design, the circumvertical modification probably is the most important maneuver for shortening vertical scars. Residual dog-ears often are excised, resulting in a short transverse scar (inverted T- or L-scar) [769]. The main indication of the circumvertical technique is the removal of 400-1000 mg of breast tissue. It is also an intermediate method and an alternative between the periareolar and the vertical techniques. The advantages of the circumvertical technique is that it is simple and fast to accomplish, only the inferior half of the gland is operated, there is no transection of the lactiferous ducts, at the end of the surgery there is a harmonious redistribution of the pleats, the vertical scar never crosses the submammary fold, and at the end of the surgery an acceptable result is almost always observed. The posterior skin retraction will improve this initial result even more. The Marcaine infiltration allows some hours of postoperative pain relief. In brief, this technique is ideal for young women because it neither distorts the remnant anatomy nor alters the future lactation, being a good alternative to moderate and large hypertrophies [770]. The patients seeking our help for breast reduction are very often young and probably planning to have children later in their lives. Therefore, it is most important to offer them a method of reduction mammoplasty that leaves as little scars and as much physiological function as possible. The vertical reduction mammoplasty is a method that leaves normal sensibility in almost all cases, the possibility of lactation, little scarring, and a pleasant form. The method can be used in all cases, ranging from mastopexy to reduction weights of over 2 kg of each breast. The vertical technique developed by 164 Ciro Comparetto and Franco Borruto Claude Lassus and Madeleine Lejour is a contemporary method of reduction that leaves few scars and conserves a maximum of physiological function. The method needs surgical skill and therefore it is not suitable for beginners in breast surgery. It is difficult to teach because it uses no patterns such as those of Strömbeck or McKissock but it gives the breasts a new form based on the anatomical circumstances and wishes of the patients [771]. The vertical reduction mammoplasty can be challenging to learn. In addition, first attempts to perform the vertical reduction mammoplasty based on a technique described by Elizabeth Hall-Findlay can lead to inconsistent aesthetic results. These problems include a persistent vertical dog-ear deformity at the nadir of the incision, a teardrop deformity of the NAC, lateral deviation of the nipple, and lateral axillary fullness [772]. For the last 20 years, D.H. Lalonde has added the No Vertical Scar breast reduction to his armamentarium of reduction techniques. This operation is almost the same as the inferior pedicle T-scar reduction. However, a small modification of the Wise pattern permits the deletion of the vertical portion of the T-scar. The first 14 years of the Dr. Lalonde’s practice consisted almost exclusively of the T-scar reduction. He currently uses the Vertical reduction, the T-reduction, and in over 150 cases, the No Vertical Scar reduction [773]. The short scar periareolar inferior pedicle reduction (SPAIR) mammoplasty is a technically straight-forward and reliable technique for managing the excessively large or ptotic breast that gives consistent and stable results over time. Advantages related to improved shapes with limited postoperative shape change should encourage the serious student of breast surgery to be familiar with the technique and concepts involved. It is recommended as an excellent option for women seeking reduction or mastopexy [774]. Procedures that minimize the resultant scars while achieving comparable results are preferred by patients and greatly enhance their overall satisfaction. Suction mammoplasty – and indeed all short-scar procedures that are performed on the breast, face, and body – rely to variable degrees on harnessing the under-appreciated capability of the skin to shrink or retract. Patients with breast hypertrophy represent a wide spectrum of deformities based on NAC size, shape, and location, skin quality and tone, and breast volume. No one breast reduction technique is ideal for reducing all breasts. Suction mammoplasty represents one of a number of methods of reduction mammoplasty that can be selected according to variations in anatomy, patient desires, and surgeon preference [775]. Breast reduction by liposuction alone is an appealing technique that has failed to gain widespread acceptance. Despite numerous studies on liposuction, the majority of surgeons remain skeptical. Two groups of patients that qualify for this procedure can be defined. However, the aesthetic result will be pleasing only for young patients. For elderly patients, liposuction breast reduction will simply achieve a weight reduction of the breast without improving the shape. Universally good liposuction results for breast weight reduction and elevation of the NAC are reported. Improvement in breast shape and correction of ptosis cannot be Aesthetic Breast Surgery 165 achieved for elderly patients. Young patients with a preoperatively pleasing breast shape can expect a preservation of the shape with the benefit of minimal scarring. Liposuction breast reduction is appealing due to selective removal of fat, ease of the procedure, and minimal scarring. The main disadvantage is that a correction of shape and ptosis is not possible with liposuction, and only young patients can expect an aesthetically pleasing result. Elderly patients may benefit from faster recovery times, a less invasive procedure, and low costs. The application of a new technique to a cancer-prone organ represents a potentially serious medical-legal issue because follow-up imaging may be impaired and a possible spread of cancer cells cannot be ruled out. Despite its technical appeal, breast reduction by liposuction alone mandates a cautious approach [776]. Therapeutic mammoplasty, the use of reduction mammoplasty and radiotherapy to surgically treat breast cancer, is an established technique for selected breast cancers and can extend the role of breast-conserving surgery. Most frequently described is the use of a wise pattern reduction for tumors that lie within the expected mammoplasty excision. However, mammoplasty techniques can be safely adapted to treat patients with cancers in all areas of the breast. Technique will vary depending upon the tumor position. Breast cancers may lie within the normal excision site of a recognized mammoplasty method (scenario A) or outside of the expected excision sites (scenario B). In scenario A, a range of recognized techniques can be performed without adaptation to widely excise the tumor and reshape the breasts. In scenario B, the techniques need to be adapted. Three decisions are needed for planning in scenario B: the skin incision, the NAC pedicle orientation, and finally the method of filling the cancer defect. The latter can be achieved by either extending the nipple pedicle or by creating a secondary pedicle within the breast dissection. Either method will move tissue that is normally excised into the cancer defect. For central tumors an inferior pedicle is usually used to both fill the defect and recreate the nipple [777-797]. Female glandular hypomastia is a frequently encountered entity that occurs either developmentally or by postpartum involution. Historically, women have long sought breast enlargement to improve physical proportions, to foster a more feminine appearance, or to enhance self-image [798]. Breast augmentation remains one of the top surgical procedures performed by plastic surgeons, probably the most commonly performed aesthetic surgical procedure. Current literature supports the concept that breast augmentation outcomes are optimized using a concept of “the process of breast augmentation.” Breast augmentation is often thought of as a surgical procedure: however, the non-surgical aspects of the procedure are more important for optimizing outcomes and minimizing reoperation and complications. The process of breast augmentation includes patient education, tissue-based preoperative planning, refined surgical technique, and defined postoperative management [799]. Choices of incisions, pocket plane, and myriad implant characteristics constitute the basis for surgical 166 Ciro Comparetto and Franco Borruto planning. Analysis of physical characteristics and inclusion of the patient in implant selection contribute to overall satisfaction and reduce requests for secondary surgery. Technical expertise in implant positioning and aseptic handling helps to prevent capsular contracture, implant malposition, and other shape problems. Despite the need for secondary surgery in some, patient satisfaction is high [800]. Driving forces behind the choice to have augmentation surgery appear to be related to feelings of low self-esteem and self-confidence. Although there is a large literature related to breast augmentation, little of it is research-based. The research that there is focuses on complications and their treatment, with an emphasis on capsular contracture. Few of the studies are long-term, although complications have been noted as long as 25 years after initial implantation. There is a need for research into the experience of women undergoing augmentation mammoplasty but, perhaps more importantly, there is also a need to examine ways in which women can be helped to accept themselves as they are. The increasing demand for reduced scars has led to the development of numerous minimal incision procedures, which constitute the most recent and exciting advances in breast surgery. Numerous different periareolar techniques are described in an attempt to eliminate scars on the breast surface by restricting them to the interface between the areola and the skin. The circumareolar mastopexy with augmentation is an excellent procedure for the right patient. Correct preoperative assessment of the patient’s skin, as well as their desires and expectations, are as critical as the surgeon’s experience and acquaintance with his own circumareolar technique. If all these factors can be brought together, however, successful operations can be performed, happy patients will ensue, and less scars will be placed [801]. These conclusions show a clear evolution of the technique as well as an improvement in the obtained aesthetic results. New techniques for periareolar mammoplasty based on repositioning the breast parenchyma and maintaining this new architecture with a stronger and more durable supporting system have been developed. The development of new materials, technical refinements in the technique, and increased experience will undoubtedly lead to even better results in the future [802]. Cosmetic breast augmentation and postmastectomy breast reconstruction surgery using synthetic implants have become established in surgical practice over more than 40 years. The operative technique for implant placement have changed somewhat during this time, as many different implant presentations have become available, but the same basic materials (polydimethylsiloxane and polyurethane) have remained in use [803]. Augmentation mammoplasty, so far, seems to be an operation with an indefinite endpoint regarding the longevity of results. It is also a procedure that has wide monetary implications and would often seem to be inappropriately used. With the advent of the latest polyurethane-covered prostheses, marketed under various trade names such as Meme, Replicon, and Optimam (Polytech, Dieburg, Germany), a more predictable result can be obtained, at least for the first six years. Much controversy still exists [804]. Aesthetic Breast Surgery 167 Polyurethane breast implants were first introduced by Ashley in 1970, with the intention of trying to reduce the high incidence of capsular contracture associated with smooth shelled, high gel bleed, silicone breast implants. The sterilization of the polyurethane foam in the early days was questionable. More recently, ethylene oxide (ETO)-sterilized polyurethane has been used in the manufacturing process and this has been shown to reduce the incidence of biofilm. The improved method of attachment of polyurethane onto the underlying high cohesive gel, barrier shell layered, silicone breast implants also encourages biointegration. Polyurethane-covered, cohesive gel, silicone implants have also been shown to reduce the incidence of other problems commonly associated with smooth or textured silicone implants, especially with reference to displacement, capsular contracture, seroma, reoperation, and biofilm and implant rupture. These findings contribute to our understanding of polyurethane implant safety, and give reasoning for a significant reduction in clinical capsular contracture rate, up to ten years after implantation, compared to contemporary silicone implants. A more permanent matrix equivalent to polyurethane may be the solution for reducing long-term capsular contracture [805]. Even if the safety of the polyurethane prosthesis has been the subject of many studies and professional and public controversies, nowadays, polyurethanecovered implants are very popular in plastic surgery for the treatment of capsular contracture. The polyurethanic capsule is a well defined foreign body reaction characterized by synovial metaplasia, a thin layer of disarranged collagen fibers, and a high vascularization. These features make possible a “young” capsule and a low occurrence of capsular contracture even over a long period (ten years). The polyurethane implants may be difficult to remove but there is no evidence that they cause an increase in the other complications: there is no evidence of polyurethane-related cancer in longterm studies (after five years). Polyurethane foam-covered breast implants remain a valid choice for the treatment of capsular contracture even if it would be very useful to verify the ease of removal of the prosthesis and to continue investigations on biodegradation products [806]. Three companies currently offer FDA-approved breast implants (Allergan, Mentor, and Sientra), but their product offerings – including permanent breast implants, breast tissue expanders, sizers, and postoperative warranty – can be difficult to compare because of brand names and company-specific jargon. The ability to have a brandagnostic understanding of all available options is important for both the surgical trainee as well as the surgeon in clinical practice [807]. In the USA, women seeking breast implants for augmentation, revision, or reconstruction can choose between saline-filled devices and round, silicone gel-filled devices. The USA has seen significant shifts in the breast implant market over the past five decades. From the moratorium on silicone gel breast implants in 1992 to their approval in 2006, there have been many developments in their manufacturing and usage. Meanwhile, saline breast implants have remained 168 Ciro Comparetto and Franco Borruto somewhat unchanged, still offering a few distinct advantages but none of the technological innovation of the silicone gel models [808]. Form-stable, highly cohesive silicone gel-filled breast implants are marketed in other countries and are currently under review by the FDA. Allergan has conducted clinical studies to investigate the safety and effectiveness of its round and anatomical (Style 410) devices for USA marketing approval. The most frequently reported complications were reoperation, implant removal with replacement, implant malposition, and capsular contracture. The FDA approved the round devices in 2006. The weight of the scientific literature suggests that silicone gelfilled breast implants do not increase a patient’s risk of cancer, autoimmune disease, reproductive effects, or suicide. As differently shaped, cohesive breast implants continue to be introduced, breast implant surgery will become more customized to the patient’s biological conditions and desires [809]. Choosing between round and anatomical formstable implants is a key decision in the process of breast augmentation. Anatomical devices have been subject to a number of misconceptions that have limited their use. However, in optimal clinical practice, the benefits and risks of both anatomical and round implants should be considered for any given patient. Patient and surgeon education is fundamental in ensuring that misunderstandings are addressed. The choice between anatomical and round devices should be based on a combination of patient desires, anatomy, and surgical history [810]. With the 2006 FDA approval of round silicone gel breast implants in the USA, there still remained a lack of versatility in implant options. The approval of Sientra’s shaped cohesive implants in 2012 brought with it the innovations needed to address varying patient needs. Because access to shaped devices is still fairly recent in the USA, some surgeons remain uncomfortable with implementing shaped cohesive gel implants into their practice. Important factors for shaped-implant selection include chest base diameter, implant height, implant volume, and implant projection for the patient’s desired outcome. With experience, surgeons will find new utility with shaped implants for a variety of patients in their practices [811]. The recent approval of MemoryShape implant by the FDA introduces a novel implant available to the surgeon for cosmetic and reconstructive breast surgery. These implants are unique due to the texture of the shell, the anatomical shape and dimensions, the degree of crosslinking of the gel, and relative form stability. The cross-linking, form stability, and cohesiveness of these implants provide surgeons with an innovative tool to more closely create a natural breast in both shape and youthful firmness [812]. Macrolane (Q-Med, Uppsala, Sweden), a compound composed of hyaluronic acid, is the newest product to be marketed for breast augmentation. Like many previous breast augmentation products, Macrolane has been authorized for use with very little published scientific data on its safety and efficacy in breast augmentation. It is strongly recommended that clinicians review the lack of data on Macrolane before offering it as a treatment option to patients [813, 814]. Aesthetic Breast Surgery 169 Breast implants are medical devices used for augmentation of the breast, to reconstruct the breast after mastectomy, and to correct breast asymmetry. The breast prostheses are likely the most frequently used medical implants. The evolution of modern silicone breast implants was not straightforward and is characterized with a lot of medical, forensic, and public attention. Currently, it is sufficiently established that implants are safe and can be used for breast reconstruction and aesthetic augmentation. Prosthesis can rupture and produce local symptoms but there is no evidence that silicone breast implants can be of any health hazards for the patients [815]. Breast implants have been used for about four decades for both reconstructive and aesthetic purposes. In 1963, the quality of the artificial implants was revolutionized by the introduction of the silicone gel-filled implant. Since, this modern prosthesis has gone through an evolution of change and improvement with several types of devices with many variations and styles within each class. Actually, for the last three decades, approximately one million women have received silicone breast implants in the USA. But, in 1992, the American FDA banned silicone from the market, leaving saline implants as the only product generally available as an alternative until now. Other filler materials were introduced in the USA and in Europe [816]. So, the silicone breast implants have recently come under scrutiny by the FDA and received much unfavorable media coverage. The gel-filled breast implants which in 1976 were “grandfathered” by the FDA have now been required to provide scientific evidence of safety and effectiveness by July 9th, 1991. The possible risks of silicone breast implants include capsular contracture, interference with early tumor detection by routine mammography, development of sarcomas in laboratory animals (no human cases are reported), silicone gel leakage, and connective tissue disease. In the less frequently used polyurethane-covered implants, the degradation of the polyurethane to diaminotoluene (TDA) has caused liver cancer in laboratory animals, yet at present, this type of implant has been voluntarily removed from the market by the manufacturer. After reviewing the available evidence, the American Society of Plastic Surgery (ASPS) still considers silicone breast implants reliable and safe [817]. Evolution of silicone breast implant design has focused primarily on advances in implant fill, surface texture, and shape. Fifth-generation, shaped, form-stable, silicone breast implants from all three major implant manufacturers are now approved for use by the FDA in the USA. As part of this approval, the FDA mandated Core Study follow-up of silicone implants for ten years after premarket approval [818]. Modern implant design has aimed to optimize aesthetic outcomes while minimizing implant-related complications, such as capsular contracture and device rupture. One of the most significant advances has been the use of highly cohesive silicone which, through extensive cross-linking, maintains its shape within the body in the presence of physiologic forces. Overall, silicone breast implants are associated with a high degree of patient satisfaction and low rates of complications. Further independent research is necessary to better establish long-term outcomes [819]. 170 Ciro Comparetto and Franco Borruto Despite the ban on silicone gel breast implants in 1992, the last decades witnessed a dramatic increase in the number of cosmetic breast augmentation procedures performed in the USA. According to the ASPS, over 132,000 women in this country underwent the procedure every year. It is estimated that they have been implanted in over two million women in the USA. This is an underestimate of the actual number of breast augmentations performed annually, as increasing numbers of non-surgeon physicians are now performing cosmetic surgery. Given the rising number of women who now seek cosmetic breast augmentation surgery, it is likely that women’s healthcare providers will be asked by their patients about breast augmentation [820]. Yet, augmentation mammoplasty is a surgical procedure with known risks and complications. Despite this, millions of women have opted to undergo the procedure. With the recent publicity surrounding this procedure, and the current concern about long-term side effects, the rationale behind women’s choice has been questioned. Often, health care professionals are less than empathetic when a woman complains about experiencing complications. This attitude seems to “blame the victim” [821]. Despite five decades of rapidly expanding application of polydimethylsiloxane as a relatively safe implantable biomaterial, the American public is being told by a vocal minority that its use in the breast implant may be dangerous. Most of the furor has been generated by consumer advocates with support of a handful of scientists who have expressed opinions, not well supported by facts, about the risks of these devices. These anxieties have been fueled in the public’s mind by a media more interested in sensationalism than disciplined reporting. The controversy has complicated the regulatory process and has become politicized in the halls of State Legislatures and Congress. Remarkably, this controversy has not involved the many other biomedical applications of silicone [822]. The FDA has required manufacturers of the implants to submit evidence of their safety and effectiveness. The implants have been shown to benefit women in psychological studies. Physical risks include capsular contracture (affecting up to half of wearers), impaired mammographic results, implant rupture, and possibly connective tissue disease. Human studies would benefit from improved research design, including controls and long-term follow-up. Professional societies of plastic surgeons, whose members benefit commercially from breast augmentation and reconstruction, have campaigned forcefully for the continued and increased use of breast implants by women [823]. Standard techniques have been used for the successful placement of mammary prostheses to enhance or replace breast tissue. All breast implants are surrounded by a capsule. The most common complication of breast implant surgery is hardening and contracture of the capsule. Explanation of implants is indicated for implant rupture, infection, extrusion, siliconoma, breast pain, painful capsular contracture, malposition, significant patient fear, and systemic symptoms thought secondary to implants. A number of alternatives are available for postexplant reconstruction, including Aesthetic Breast Surgery 171 myocutaneous flaps and free tissue transfers [824]. Several alterations to both elastomer shell and filler gel have been made over the years to improve silicone gel implants ability to replicate the natural breast and to decrease the incidence of capsular contracture. The latter is a pathological process involving the periprosthetic tissues formed in response to the presence of the implant. When severe, capsular contracture may cause firmness, distortion, and pain [825]. In the past 30 years, there have been multiple published reports associating silicone breast implants with scleroderma, morphea, systemic lupus erythematosus (SLE), rheumatoid arthritis, calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia (CREST) syndrome, and the socalled “human adjuvant disease.” The alleged offending material, silicone, is a synthetic polymer containing a silicon-oxygen backbone. Beginning with the heating of silicon dioxide (SiO2) in the presence of carbon, elemental silicon is produced. Methylchloride is added and the resulting product is hydrolyzed to form low molecular weight prepolymers which are linked to form linear silicone polymers and cross-linked to yield silicone rubbers or elastomers. The polymeric and hydrophobic characteristics of silicone and the presence of electrostatic charges and organic sidegroups make silicone a potentially ideal immunogen, leading to cross-reactivity with autoantigens. Silicon is an essential constituent of proteoglycans which theoretically could result in immunological cross-reactions between silicone and connective tissues. Although the literature contains numerous examples of silicone-associated autoimmune disease, there is no consistent pattern of immunological abnormalities observed. There are, however, some intriguing and interesting observations [826]. Understanding the immunology of silicone breast implants, at present, consists of reconciling an increasingly large body of data with an older, established, but otherwise unsubstantiated theory. Despite the variety of silicone breast implants, there are nevertheless certain phenomena that occur with all silicone gelfilled devices. Recent clinical findings suggest that in some patients, silicone will act as an adjuvant on native macromolecules and render them immunogenic. Further largescale studies are needed to determine if a link between silicone exposure and autoimmunity exists. Also, since the inducing events of autoimmune diseases are unknown, studies on silicone could provide a model for autoimmune diseases associated with toxicological factors [827]. Many implants may need in-vivo evaluation by imaging, particularly if they lead to complications and/or clinical symptoms. They can also pose problems in the assessment of surrounding breast tissue by conventional mammography. In this respect, imaging modalities such as ultrasound, CT, and MRI offer greater possibilities to assess a failing implant, as well as surrounding breast tissue [828]. All radiologists need to be able to recognize the normal appearances of the more commonly used implants on various imaging modalities, and breast radiologists in particular are facing new challenges when imaging the women involved [829]. There are some reports suggesting that implants can 172 Ciro Comparetto and Franco Borruto interfere with mammography and may lead to delayed breast cancer diagnosis, even when implant-displaced mammography (Eklund technique) is performed. The augmented breast is a challenge to the mammographer. Many of the palpable and mammographically detected abnormalities in these patients are related to the implant itself. Since, however, there is breast tissue present with cosmetic augmentation, the full range of fibrocystic and neoplastic conditions that can affect the breast may be seen. The presence of the implant makes imaging the breast more difficult because the implant obscures the nearby breast tissue [830]. Recent reports suggest that screening mammography may not be appropriate in augmented women, but breast self-examination may be effective in these women. Ultrasonography may be useful in screening augmented women without risk of rupturing the implant. In appropriate cases, MRI should be considered as an adjunct to mammography and ultrasonography. The question of whether augmented women should not undergo CNB because of the possibility of damage to the implant should be considered. The challenge of the screening and diagnosis of breast cancer in augmented women is important in order to detect more of their cancer at a preclinical stage, because we can expect to see breast cancer in augmented women with increasing frequency over the next decades [831]. MRI has proved to be an excellent imaging modality in locating free silicone and evaluating an implant for rupture, with a sensitivity of approximately 94% and specificity of 97%. Silicone has a unique MRI frequency and long T1 and T2 relaxation times, which allows several MRI sequences to provide excellent diagnostic images. The most commonly used sequences include T2-weighted, short-TI inversion recovery (STIR), and chemical shift imaging. The T2-weighted and STIR sequences are often used in conjunction with chemical water suppression. The most reliable findings on MRI for detection of implant rupture include identification of the collapsed implant shell (linguine sign) and free silicone within the breast parenchyma [832]. The MRI appearances of various complications of polyacrylamide gel injection mammoplasty include breast asymmetry, intramammary or extramammary gel displacement, including intrathoracic extension, and glandular atrophy, inflammation, and infection resulting in mastectomy. Because polyacrylamide gel has a high water content, it has been found that sagittal and axial T2-weighted are the best sequences to use to detect complications [833]. MRI is the primary modality for assessing the integrity of silicone implants and has excellent sensitivity and specificity, and the FDA currently recommends periodic MRI screening for silent silicone breast implant rupture. Familiarity with the types of silicone implants and potential complications is essential for the radiologist. Signs of intracapsular rupture include the noose, droplet, subcapsular line, and linguine signs. Signs of extracapsular rupture include herniation of silicone with a capsular defect and extruded silicone material. Specific sequences including water and silicone suppression are essential for distinguishing rupture from other pathologies and artifacts. MRI provides valuable information about the integrity of silicone implants and associated Aesthetic Breast Surgery 173 complications [834]. Although there are many 3-D imaging systems currently available on the market, all of them require a high degree of interaction on the part of the user, making them clinically impractical. Moreover, though claims have been made regarding the validity of these systems for imaging the breast form, there have been no previous reports validating any commercially-available implant simulation models in the plastic surgery literature. A novel 4-D (automated 3-D) breast imaging system has been validated as an evidence-based simulation tool for patient consultation, surgical planning, and outcomes analysis in cosmetic breast augmentation. Based on a series of longitudinal correlation studies with several patient cohorts, a highly statistically significant degree of correlation and reliability between the automated measurements obtained with the 4-D system and manual measurements has been found [835-837]. No association has been found between the presence of breast implants in a breast and an increased risk of breast cancer. However, as the population of women with breast implants ages, an increasing number of them will develop breast cancer: a reflection of the fact that the incidence of the disease increases with increasing age. Debate continues on the effect of breast implants on the efficacy of mammography in diagnosing breast cancer, and the role of other imaging techniques for this purpose, as well as the limitations that the presence of implants place on percutaneous biopsy techniques [838]. The majority of women receiving breast augmentation surgery do so at a time in their lives when both reproduction and lactation are common. It does not occur to most women to consider the possible effects breast augmentation surgery may have on their future ability to exclusively breast-feed their baby. Most women raise concerns about their inability to exclusively breast-feed years after surgery when they have a child. It is therefore important that women considering breast augmentation surgery be fully informed of the possible effects surgery may have on their future ability to lactate. Women who undergo breast augmentation surgery have a greater incidence of lactation insufficiency. Factors directly related to the surgical procedure as well as short- and long-term complications of surgery compromise future ability to exclusively breast-feed a baby. Factors directly related to surgery include severing of the lateral and medial branches of the fourth intercostal nerve or the nerve endings of the NAC, which lead to reduced sensation and loss of the suckling reflex resulting in decreased milk production. Hematoma formation increases the risk of developing capsular contracture therefore necessitating the need for further surgical intervention. Infection also requires further intervention and, as a result, further risk to the breast tissue. Long-term breast pain, capsular contracture, and pressure effects on the breast from the implant are all possible long-term complications that compromise a woman’s future ability to lactate and exclusively breast-feed her baby. With good surgical technique and proper postoperative management, most of the complications associated with surgery that may result in insufficient milk production can be minimized but not always avoided. Compared with 174 Ciro Comparetto and Franco Borruto non-augmented women, women who have had augmentation surgery have a higher incidence of lactation insufficiency [839]. Far more information is available about the relationship of breast reduction surgery and lactation than about breast augmentation surgery and lactation. Women are often able to breast-feed after breast augmentation surgery. Depending upon the type of breast reduction surgery, estimates of the number of women who can lactate after reduction surgery range from 0-70% [840]. Women who have had breast surgery are likely to have many questions and misgivings about their ability to breast-feed. In order to answer the woman’s questions and provide her with supportive and guiding care, the lactation consultant must understand the nature of the surgery, the reasons for it, and the likely outcome with respect to the ability to lactate [841]. Although breast prosthesis implantation is the most common technique of cosmetic breast augmentation, other unusual techniques such as autologous fat implantation as well as direct liquid silicone and paraffin injections have also been used [842]. Since the 1980s, there has been an increased interest in autogenous fat grafting for breast augmentation. However, concerns over graft survival and interference with breast cancer screening have limited its application. Autologous fat grafting is an increasingly popular procedure used for facial rejuvenation and body contouring. A review of the literature demonstrated that there is no standard test for determining fat viability or volume augmentation after grafting. Furthermore, there is no difference in cell viability seen between syringe aspiration and liposuction pump aspiration harvest techniques. The decision to wash or centrifuge the fat plays very little role in fat graft survival. There is no difference between cell viability as a function of harvest location. Nearly all studies show no significant effect of local anesthesia on adipocyte cells. There are excellent data that support the fact that low-shear devices maintain fat structural integrity. There is quality evidence that supports longevity of fat grafted to the breast. Two studies support large-volume fat grafting longevity but fail to prove their results using objective measures or with sufficiently large sample sizes. External pre-expansion devices improve total graft survival rate. There is quality evidence to support that fat should be injected soon after harvesting, as properties of fat begin to change after processing. Microneedling (preconditioning) before fat grafting has been demonstrated to improve fat survival. Currently, the highest levels of evidence derive from human studies of clinical trials and animal studies using human fat. The evidence helps to address the need for accurate and quantitative viability assays. These assays would facilitate a systematic evaluation of each procedural step during fat graft harvest, processing, and grafting to improve the overall viability and predictability of fat grafts [843]. As the technique of autologous fat grafting is being refined and perfected, its clinical applications are expanding. The use of autologous fat grafting for primary breast augmentation is controversial due to a lack of clarity regarding its safety and efficacy. Most notably, Aesthetic Breast Surgery 175 concerns about interference with the detection of breast cancer have been raised, but these have not been clearly addressed in the literature [844]. Recent technical advances in fat grafting and the development of surgical devices such as liposuction cannulae have made fat grafting a relatively safe and effective procedure. However, new guidelines issued by the ASPS in 2009 announced that fat grafting to the breast is not a strongly recommended procedure, as there are limited scientific data on the safety and efficacy of this particular type of fat transfer. Recent progress by several groups has revealed that multipotent adult stem cells are present in human adipose tissue. This cell population, termed ASC, represents a promising approach to future cell-based therapies, such as tissue engineering and regeneration. In fact, several reports have shown that ASC play a pivotal role in graft survival through both adipogenesis and angiogenesis [845]. Since its introduction, refinements in harvesting and grafting techniques have improved results. The available literature consists primarily of case reports and series. There are no RCT, and outcomes thus far have not been measured in a standardized way. The limited data relating to breast cancer screening did not note a significant interference. Concerns have been raised that the placement of mature adipocytes and ASC into the hormonally-active environment of the breast may potentiate breast cancer, but there are no RCT that investigate this possibility and a consensus regarding the basic science is still developing. Large multicenter, prospective RCT are necessary to further investigate the many issues relating to the application of autogenous fat grafting for augmentation of the breast [846]. So, the use of autologous fat transfer for breast augmentation is still controversial due to ongoing concerns regarding its efficacy and safety, most notably, concerns about breast cancer risk and detection. Autologous fat transfer for breast augmentation is not yet standardized. Therefore, outcomes vary widely depending on the surgeon’s expertise. The majority of reported complications are of low morbidity, and based on available data, the procedure has a good long-term safety profile. Although there is no evidence that fat grafting increases breast malignancy risk, long-term followup is required [847-852]. Revisionary breast surgery in previously augmented patients is complex, with many variables that have to be considered. Obtaining durable repairs is challenging because these patients often present with thinned breast tissue, inadequate local tissue, and/or scarred breast envelope from multiple procedures. Capsular contracture, ptosis, tissue atrophy, and wrinkling/rippling are some of the most frequent reasons for reoperation. Conventional repair techniques generally involve a combination of capsule modification (capsular flaps), site change, mastopexy, and implant exchange. Recently, ADM have been introduced into revision surgery to reinforce soft tissue, reinforce the implant pocket, and potentially mitigate capsular contracture [853, 854]. Simple application of negative suction drains through the umbilicus during transaxillary breast augmentation obtained a more natural drainage and an easier positioning of the drain at the dependant 176 Ciro Comparetto and Franco Borruto portions than placement of the drains at the transaxillary incision site. Moreover, the patient was more satisfied due to increased comfort while wearing clothes, which resulted in a quicker recovery to everyday activities. In addition, the scar of the drain site was hidden on the inner side of the umbilicus [855-857]. Many of these patients present with secondary problems or complications following their primary breast augmentation. Two of the most common complications are capsular contracture and implant malposition. Familiarity and comfort with the assessment and management of these complications is necessary for all plastic surgeons. An up-to-date understanding of current devices and techniques may decrease the need to manage future complications from the current cohort of breast augmentation patients [858, 859]. Abdominoplasty and breast surgery are frequently appealing to patients as combined procedures. The practice of combining abdominoplasty with other procedures originates from abdominoplasty performed in conjunction with intra-abdominal or gynecological surgery. Initially, the focus of combined surgery was on ensuring safety and minimizing local (e.g., wound healing) complications. As surgeons began combining abdominoplasty with distant procedures such as breast surgery, because the individual procedures have little adverse impact on one another and are not altered because of the combination, concerns with systemic morbidity surpassed the initial focus on avoiding local complications. Prevention of venous thromboembolism became a paramount concern. Some surgeons perform abdominoplasty in conjunction with other procedures more frequently than in isolation, reflecting broader societal demand. Indeed, because of the effects of pregnancy and aging, abdominoplasty is being performed in conjunction with breast surgery with frequency sufficient to have driven the term “mommy makeover” into mainstream parlance. Consideration regarding length of surgery and the other recommendations allows for the safe and successful execution of this common combination [860]. Deformities caused by partial mastectomy can often be helped by plastic surgical techniques. Immediate repair of partial mastectomy defects is especially useful when skin, as well as breast tissue, has been resected. Distant tissue can be used, but most partial mastectomy defects are best repaired with local flaps. Shifting the defect into the axilla, where it is less noticeable, is often helpful. Alternatively, the breast can be reshaped using techniques similar to those used in reduction mammoplasty. In that situation, reduction of the opposite breast often is indicated for symmetry [861]. Fundamental principles of management of breast burns begin with recognition and preservation of any viable breast bud tissue. Reconstruction begins when the burned breast envelope is insufficient to allow unrestricted breast development. Complete contracture release is obtained by incision or excision of the restricting burn scar and thick split-thickness grafting. Occasionally, breast mound reconstruction with regional musculocutaneous flaps or tissue expanders is necessary. Balancing procedures, such as reduction or mastopexy of an opposite unburned breast, are often helpful. After a period Aesthetic Breast Surgery 177 of six to 12 months of compression garments, scar management, and settling, NAC reconstruction is undertaken and consists of a combination of local flaps, full-thickness grafting, or composite grafts tailored to each patient’s needs. Long-term follow-up is necessary to ensure that breast development continues satisfactorily and that contractures do not recur [862]. One positive consequence of the challenge to silicone breast implants has been renewed interest in the psychological dimensions of plastic surgery. When asked questions about the psychological outcomes of women with breast implants, plastic surgery responded with work that is changing the very framework on which concepts such as body image and quality of life are founded. In the course of exploring the psychological impact of breast augmentation, traditional ways of thinking about patient characteristics and motivations have been called into question. There is a new focus on evidence-based outcomes research and an active search for methods that are valid, reliable, and sensitive enough to recognize and measure the emotional impact of changing physical appearance. With more information about psychiatric comorbidities and the identification of variables that influence patients’ attitudes, augmentation mammoplasty with implants is better understood. Meanwhile, a new generation of investigators has been stimulated to study and reinterpret the psychodynamics of the aesthetic surgery experience [863]. The psychological aspects of cosmetic breast augmentation have been the focus of a great deal of empiric study over the past 50 years. Methodological limitations of the studies investigating the preoperative psychosocial status of breast augmentation candidates make it difficult to draw firm conclusions about the potential psychological differences between these women and those not interested in breast augmentation. Postoperative satisfaction rates are high, and several studies suggest that patients experience improvements in body image following surgery. The effects of breast augmentation on other areas of psychological functioning are less clear. Based on the epidemiological studies published to date, the suicide rate among women with cosmetic breast implants is two to three times the expected rate. The literature in this area should be used to guide the psychosocial assessment and management of cosmetic breast augmentation patients. There currently is little evidence to support a recommendation that all women who present for cosmetic breast augmentation be required to undergo a psychiatric evaluation before surgery. Given the relationship between breast implants and suicide, however, it is recommended that women with a history of psychopathology who present for breast augmentation, or those who are suspected by the plastic surgeon of having some form of psychopathological abnormality, should undergo a mental health consultation before surgery [864]. In conclusion, when silicone gel breast implants became the subject of a public health controversy in the early 1990s, the most pressing concern was safety. Another, less publicized issue is the need for implants. Stakeholders in this history constructed 178 Ciro Comparetto and Franco Borruto need as legitimized individual desire, the form of which shifted with changes in the technological and social context [865]. Breast augmentation remains a popular aesthetic procedure. Even with the current negative publicity, breast augmentation continues to be a widely accepted procedure. The materials that make up the breast implants have been in use for over 40 years with only minimal complications. Scar contractures continue to be the major complication with this surgery. A causal relationship between silicone breast implants, neoplasms of the breasts, or autoimmune diseases have not been demonstrated in experimental studies on humans. Scientific data presented to the FDA will determine if silicone-containing products will continue to be used for implantation. There is enough concern among some physicians and patients that many are now turning to saline implants. Some of the patients do not entertain the idea of saline implants because of the incidence of deflation and reoperation [866]. The silicone breast implant controversy has amassed a great deal of media coverage in the past years. Unfortunately, separating fact from fiction has been extremely frustrating and difficult, not only for physicians but for women who have either had or are considering cosmetic or reconstructive surgery of the breast. Breast implants are placed not only by plastic and reconstructive surgeons, but by otolaryngologists, “facial plastic surgeons,” obstetricians/gynecologists, general surgeons, dermatologists, and family practitioners. It is the ethical and legal responsibility of the physicians who elect to perform these procedures to provide adequate care and follow-up for these patients when either real or perceived problems arise. Accurate information, reassurance, and occasionally reoperations are required for many of these patients [867]. There are informational gaps, but not all of these can be laid at the door of imperfect studies or failed scientific methods. Certain properties of the implants are still unmeasurable, immunological investigation is still evolving, the cause of wound contraction is inexplicable here or in the burnscar contracture, and the indications for and results of this surgery necessarily are subjective. Still, there are a number of investigative avenues open to us, and our cumulative experience shows no reluctance on the part of plastic surgeons to initiate further studies [868]. Breast enhancement through augmentation improves not only the woman’s physical appearance but also contributes to her psychological well-being. With the current emphasis placed on women’s breasts in the media, it is not surprising that small-breasted women feel inadequate. Recent FDA approval of saline implants has given them a new image, and more women are seeking breast augmentation. As long as the woman understands that this operation has associated risks, a physician-patient relationship may be developed resulting in many years of happiness and increased self-esteem. The shape, contour, and size of a woman’s breasts are permanently altered by augmentation mammoplasty and breast reduction. Although each case is unique, the outcome of each procedure should result in a satisfied patient [869]. Mankind remains infatuated with Aesthetic Breast Surgery 179 finding the “fountain of youth,” and plastic surgery has become a very important component in the search for eternal youth. Invasive procedures such as face-lifts, body contouring, and implantation of silicone mammary implants as well as less invasive procedures such as wrinkle decreasing protocols using filler substances or botulinum toxin, effectively reshape and rejuvenate the aging face or body. However, despite the improved cosmetic appearance of the individual, these treatments disrupt normal aging processes on cellular and molecular level. For example, silicone degradation products promote protein denaturation and activate cells of both the innate and adaptive immune system, thus perpetuating a chronic proinflammatory response of the local tissue [870]. Nevertheless, breast augmentation remains one of the most common aesthetic procedures performed in the USA. Silicone implants have undergone an evolution with the availability of both fourth- and fifth-generation devices from the three leading manufacturers in the USA. Clinicians should strive to provide ongoing data and sound science to continue to improve clinical outcomes in the future [871]. The future may hold promise for the introduction of new implants for the augmentation of the breast and for reconstruction. The constant demand and pressure placed on the manufacturers to keep developing new fillers are proof that satisfaction with saline implants is lacking. Today, however, the saline implant may be believed to be the safest breast implant available for clinical application. The demand necessitates that implants be used for enhancement of a woman’s self-image and for reconstruction. The smooth-surface implants are considered more popular and safer for patients, perhaps because it has been well documented that the shell may have been the cause of the problem. However, the constancy in holding on using and reapplying the same principles of a faulty shell may not be as scientific an endeavor as we have done in the past, and to keep placing different fillers in the faulty shell. Perhaps the lamination process used in manufacturing, the air in the shell, or wear and tear produce mechanical weakening of the shell that eventually will lead to loss of integrity of the silicone-shell breast implant. The global community is looking to plastic surgeons for a solution. The applications and the demand for breast implants are global in nature. Meanwhile, as clinicians are waiting for a new implantable breast device, they will continue to use what is available and advise patients that the implantation of a breast prosthesis is not a life-long endeavor. There is a need, however, for the implants to be maintained by the process of exchange every eight to ten years. Breast reaugmentation is to be performed as a standard because plastic surgeons will be waiting for further clarifications from the regulators or the scientific community. The goal is to produce a good outcome and maintain safety for the patients with a high standard of care [872]. The breast implant issue is a “bad news/good news” story. For many women with implants, the controversy has caused a fair degree of anxiety which may or may not be resolved as further information becomes available. It has also taken its toll on Dow Corning. Whole lines of medical products have been 180 Ciro Comparetto and Franco Borruto eliminated or are being phase out. The development of new medical applications has been terminated. As a consequence, employees have lost their jobs. What the effect will be on the biomedical industry as a whole remains to be seen. While silicones have been an important component in various medical devices, it is likely that other materials can be used as replacements. However, suppliers of non-silicone materials are also reevaluating their role in this market. For example, Du Pont, the nation’s largest chemical company, has determined that the unpredictable and excessive costs of doing business with manufacturers of implantable medical devices no longer justifies the unrestricted sale of standard raw materials into this industry. Other companies are quietly following suit. On the up side, it is possible that the research being driven by this controversy will result in a greater understanding of the immunological implications of xenobiotics, of the importance of non-biased observations, of the need for ready access to valid data sets, and of the opportunity for valid scientific information to guide legal decisions. Only time will tell [873-875]. Chapter XI Breast Surgery Complications Most breast operations are categorized as low-morbidity procedures, but a variety of complications can occur in association with diagnostic and multidisciplinary management procedures. Some of these complications are related to the breast itself, and others are associated with axillary staging procedures. Some of them are general, nonspecific complications (wound infections, seroma formation, and hematoma). Other complications are specific to particular breast-related procedures: lumpectomy (including both diagnostic open biopsy and breast-conservation therapy for cancer), mastectomy, ALN dissection, lymphatic mapping/SLNB, and reconstruction [876]. Over the past three decades, breast-conservation therapy with lumpectomy and WBI has become a standard option for the majority of women with newly diagnosed breast cancer. Long-term local control is achieved in approximately 85% of patients, and the therapy is generally well tolerated. There can, however, be long-term effects on the breast and other nearby tissues that may range from asymptomatic findings on examination to severe, debilitating problems. Infection, fat necrosis, and severe musculoskeletal problems such as osteoradionecrosis or soft-tissue necrosis are uncommon, affecting less than 5% of patients. However, changes in range of motion, mild-to-moderate musculoskeletal pain, and arm and breast edema are much more common. As more women choose breastconservation therapy for management of their breast cancer, physicians will encounter these problems, as well as in-breast tumor recurrence, with greater frequency [877]. Upper-extremity pain is a common and debilitating problem for patients with breast cancer. Although there is considerable literature describing symptoms, little is available on the specific disorders responsible for pain and debility in these patients. Cervical radiculopathy, brachial plexopathy, neuropathy, rotator cuff tendonitis, adhesive capsulitis, epicondylitis, postmastectomy syndrome, swelling, and bone metastases are 182 Ciro Comparetto and Franco Borruto among the common disorders responsible for upper-extremity pain in breast cancer patients [878]. Needle biopsy of the breast is widely practiced. Imaging-guidance ensures a high degree of accuracy. However, sporadic cases of disease recurrence suggest that in some cases the procedure itself may contribute to this complication. There is histological evidence of seeding of tumor cells from the primary neoplastic site into adjacent breast tissue following biopsy. However, as the interval between biopsy and surgery lengthens then the incidence of seeding declines, which suggests that displaced tumor cells are not viable. Clinical recurrence at the site of a needle biopsy is uncommon and the relationship between biopsy and later recurrence is difficult to confirm. There is some evidence to suggest that cell seeding may be reduced when vacuum biopsy devices are deployed [879, 880]. Pneumothorax is a rare but recognized complication of diagnostic FNA of the breast. The reported incidence of pneumothorax after diagnostic aspiration of the breast in seven series varied between three in 100 and one in 10 000, but the weight of evidence tended towards the latter rate. Two studies reported that the complication is more common in the hands of trainees. It is not always possible to maintain the aspirating needle parallel or tangential to the chest wall. Pleural puncture may be more common than is apparent, and is most common in the tail of the breast in a thin woman. It is important that breast clinicians are aware of the risk of pneumothorax but, provided proper care has been taken, this complication is not the result of a negligent act [881]. In animal tumor systems, all three major treatment modalities, surgery, radiotherapy, and chemotherapy, may increase the incidence of metastases in the presence of circulating viable tumor cells. In breast cancer patients, selected studies can be found which report an increased incidence of metastases after surgery, radiotherapy, or chemotherapy, but these effects appear to exert little influence on overall survival. Caution is advised in using systemic therapy prior to effective primary tumor cytoreductive treatment. Minimal surgery, loco-regional radiotherapy, and effective adjuvant systemic therapy may result in the improved survival of patients with breast cancer with minimal functional or cosmetic impairment [882]. There is great interest among oncologists concerning what we might learn by examining the pattern of relapse after breast cancer surgery. What you see depends upon how hard you look. Up to now, investigators have examined the hazard ratio for relapse every 6-12 months. In a research paper, the Milan group have looked at the hazard ratio every three months and have found, for the first time, a distinct, very early peak of relapse in a group of premenopausal, node-positive patients not given chemotherapy or hormone therapy [883]. Thus, the act of surgery can provoke the outgrowth of dormant micrometastases, which often leads to the failure of screening to deliver its promise. The therapeutic consequence of this idea involves the use of antiangiogenic drugs before surgery [884]. Dormant breast cancer cells are a reality that cannot be overlooked. They may stay Breast Surgery Complications 183 dormant either after a spread of cancer cells caused by surgery or after being generated by spontaneous or induced mutations in the course of breast gland growth [885]. For various reasons, it is believed that the short postoperative period is critical for eliminating minimal residual disease, thus markedly impacting long-term survival. Unfortunately, both animal and human studies have shown that surgery induces suppression of antimetastatic cell-mediated immunity at this critical period, which is suggested to worsen patients’ prognosis. The use of prophylactic interventions feasible in cancer patients to avoid postoperative suppression of cell-mediated immunity has been shown to significantly reduce postoperative metastasis in animal models, including mammary adenocarcinoma, and initial data suggest similar efficacy in breast cancer patients. Prophylactic interventions can easily be applied by health-care practitioners and hold promise in reducing long-term recurrence and metastasis in cancer patients [886]. To explain bimodal relapse patterns observed in breast cancer data, it has been proposed that metastatic breast cancer growth commonly includes periods of temporary dormancy at both the single cell phase and the avascular micrometastasis phase. The half-lives of these states are one and two years, respectively. It has also been suggested that surgery to remove the primary tumor often terminates dormancy resulting in accelerated relapses. These iatrogenic events are very common in that over half of all metastatic relapses progress in that manner. Assuming this is true, there should be ample and clear evidence in clinical data. Dormancy can be identified in these diverse data but most conspicuous is the sudden escape from dormancy following primary surgery. These quantitative findings provide linkage between experimental studies of tumor dormancy and clinical efforts to improve patient outcome [887-889]. Loco-regional failures after primary treatment for breast cancer include a diverse group of lesions that represent different categories of failures with various prognoses. Although patients with chest wall recurrences and regional nodal failures after traditional radical surgery have a poor prognosis, many patients can still achieve a significant degree of palliation and even long-term survival or cure with carefully orchestrated multimodal treatment. In patients who have breast failures after breast-conservation surgery and radiation, long-term salvage and cure can be achieved for the majority with prompt detection and appropriate treatment, which, like treatment for primary breast cancer, includes a consideration not only of local control but also of the risk of subsequent systemic failure and its need for treatment [890]. Despite the success of both breast-conserving surgery and mastectomy, some women will experience a loco-regional recurrence of their breast cancer. Predictors for loco-regional recurrence after breastconserving therapy or mastectomy have been identified, including patient, tumor, and treatment-related factors. The role of surgery, radiation, and chemotherapy as treatment has evolved over time and many patients now have the potential for salvage after locoregional recurrence. In those instances where evidence is lacking or not definitive, expert 184 Ciro Comparetto and Franco Borruto opinion may be used to recommend imaging or treatment [891]. Loco-regional relapse after breast-conserving surgery and radiation therapy is operable and not associated with concurrent distant metastases in most cases. Salvage mastectomy results in loco-regional control for most patients. The extent of the surgery relates to the extent of the locoregional recurrence and does not carry an increased complication rate. The outcome of salvage mastectomy depends on the disease-free interval from initial breast-conserving surgery and radiation therapy to loco-regional recurrence. Additional factors, such as the extent and histological type of the recurrence, as well as the ALN status, either at the time of initial breast conservation or at salvage mastectomy, may influence outcome and require further study [892]. SLNB became the standard of care before consensus on the technique was reached and without RCT having shown a similar or decreased axillary recurrence rate. Uni- and multivariable analyses showed that the lowest recurrence rates were reported in studies performed in cancer centers, in studies that described the use of 99m Tc-sulphur colloid, and also when investigators used the superficial injection technique or evaluated the harvested SLN with H&E and IHC staining (p < 0.01). The axillary recurrence rate in SLN-negative patients is 0.3%, which is well within the desired range. The median sensitivity of the procedure appears to be as high as 100%. The recurrence rate is influenced by the differences in the lymphatic mapping technique [893, 894]. Breast-conservation therapy has gained acceptance as treatment for limited disease due to breast cancer. Unfortunately, a minority of patients who undergo this therapy will develop cellulitis of the breast, often recurrently, months to years later. A definitive pathogen has not been identified in the large majority of cases reported to date. Whilst some patients develop systemic toxicity with local skin changes of cellulitis, others manifest no fever, chills, or leukocytosis. Local breast findings gradually clear with antibiotic treatment: when breast changes persist, non-inflammatory causes, including tumor recurrence, of the breast should be considered. More study is needed to define risk factors for the development of breast cellulitis complicating breast-conservation therapy [895]. The clinical scenario of upper extremity cellulitis after axillary dissection for breast cancer mimics the presentation of cellulitis in the lower extremity. Until diagnostic methods or treatment advances can eliminate the indications for axillary lymphadenectomy, many women treated for breast cancer will be at long-term risk for the development of cellulitis due to localized immune impairment. Patient and physician awareness of this syndrome is the best available tool to prevent secondary exacerbation of lymphedema. Prompt treatment with appropriate antibiotics appears universally successful. Antistreptococcal antibiotics should not be withheld pending results of blood or tissue cultures, since in only a few cases a pathogen will be isolated. Although there are no studies confirming the concept, it is likely that appropriate treatment for lymphedema may reduce the risk of infection [896]. Breast Surgery Complications 185 Seroma is a common problem following breast cancer surgery causing patient discomfort and prolongation of hospital stay [897]. Breast seromas are tumor-like collections of serosanguineous fluid in breast tissue that occur following excision biopsy, lumpectomy, mastectomy, and plastic surgery procedures such as augmentation, prosthesis explantation, breast reduction, and breast reconstruction. Mammographically seromas are water-density masses located at the surgical site. They exhibit features characteristic of fluid collections on sonographic evaluation [898]. Seromas are the most frequent complications following breast surgery, resulting in significant discomfort and morbidity with possible delays in commencing adjuvant therapies. Varied clinical practices exist in the techniques employed to prevent and manage seromas. Although prevention is the best strategy, seromas remain problematic. Prevention is key to the management of seromas. Methods employed to prevent seromas include suction drainage, shoulder immobilization, quilting sutures, fibrin sealants, and innovative measures of managing the axilla, among others. The evidence demonstrated that a combination of quilting and drains significantly reduces the incidence and volumes of seromas. These effects are sustained by minimizing use of electrocautery, alongside increasing frequencies of ASLNB and node sampling. The efficacy data on fibrin sealants is inconclusive and consequently should not be routinely used alone or accompanied by quilting sutures. Clinically significant seromas deemed “symptomatic” by patients and complicating infected seromas should be aspirated. There are limited data on the recommended treatment of established seromas with a paucity of high-quality studies and further research involving RCT are indicated [899]. Seroma, as a complication of prosthetic breast reconstruction, results in patient distress, increased office visits, undesirable aesthetic outcomes, and – importantly – may escalate to infection and frank prosthesis loss. Seromas following prosthetic breast reconstruction are complicated by the hypovascular, proinflammatory milieu of the mastectomy skin flap, the geometrically complex dead space, and the presence of a foreign body with potential contamination and biofilm. There is reasonable evidence to suggest that these factors contribute to a progression of seroma to infection and prosthesis loss [900]. Postoperative swelling following prosthetic implant breast augmentation and reconstruction is not uncommon. Prompt diagnosis and targeted treatment are critical. Current treatment recommendations achieve a diagnosis using specialized equipment with needle-guided imaging and/or surgical modalities. These techniques are expensive and delay diagnosis and treatment. Some surgeons use an in-office, non-imaging technique to drain periprosthetic fluid after unilateral breast swelling after breast reconstruction or augmentation. This technique is effective in diagnosing and treating seroma fluid with minimal risk of implant damage or perforation [901, 902]. Upper extremity lymphedema is a relatively frequent complication following the management of breast cancer [903]. About one-third of all women treated for breast 186 Ciro Comparetto and Franco Borruto cancer develop arm lymphedema. In addition to the worry of the cancer itself, the swollen and heavy arm is both a physical and a psychosocial handicap for the patients. The incidence of lymphedema does seem to be reducing with modern approaches to breast cancer management. Axillary treatment seems to determine its development but more studies looking specifically at this problem are needed [904]. Lymphedema is often accepted as an inevitable and untreatable common consequence of breast cancer management. There has been little investigation of its pathophysiology, but reasonable hypotheses can be elaborated based on the known physiology of lymph production and removal. Chronic edema of the arm (postmastectomy edema) often develops without warning months or years later. Although the original cause of postmastectomy edema is damage to axillary lymph drainage routes by surgery and radiotherapy, many observations suggest that additional factors are involved. Recent attention has focused on the Starling forces in the skin and subcutis in postmastectomy edema. An important finding was that the protein concentration, and hence colloid osmotic pressure, of the subcutaneous interstitial fluid of the postmastectomy edema arm is unexpectedly lower than in the unaffected arm, correlating negatively with the degree of swelling. There are several possible explanations for this, such as a rise in capillary filtration rate, or interstitial proteolysis. A systemic component to postmastectomy edema is suggested by the finding of a lower plasma protein concentration in affected women compared with a matched postmastectomy group without swelling. A recent study using intravital capillaroscopy has indicated that angiogenesis occurs in the skin in postmastectomy edema, and an increased capillary surface area for filtration could result in an increased fluid load on a compromised lymph drainage system. Further elucidation of the pathophysiological processes in postmastectomy edema, in particular the adjustments to Starling forces, will enable more effective therapy of this distressing condition [905]. Lymphedema is generally described by arm swelling and dysfunction. Both diagnosis and treatment remain largely empirical. A large clinical experience suggests that outcome is best with a multidisciplinary team approach that is based in an outpatient setting and combines gradient compression garments, sequential pneumatic pumps, and ancillary support services. Through this program, a majority of patients see greater than 50% reduction in their lymphedema [906]. Previous surgical and conservative treatments have not always given satisfactory and permanent results, conceivably because lymphedema causes hypertrophy of the subcutaneous adipose tissue. From this point of view, liposuction combined with controlled compression therapy is an interesting approach, as the hypertrophied adipose tissue is effectively removed and the outcome sustained by wearing a compression garment. The use of a compression garment after liposuction is necessary in order to maintain the normalized arm volume [907]. Physiotherapy contributes to its treatment, using different techniques that have been developed over the years. Physiotherapy resources used for lymphedema treatment Breast Surgery Complications 187 include complex decongestive therapy, pneumatic compression, high-voltage electrical stimulation, and laser therapy. The literature shows that better results are obtained with combined techniques. Complex decongestive therapy is the most used protocol, and its association with pneumatic compression has demonstrated efficacy. The new techniques high-voltage electrical stimulation and laser present satisfactory results [908]. Clinical recommendations guide survivors to avoid the use of the affected arm. This may lead to deconditioning and, ironically, the very outcome women seek to avoid. Recently published studies run counter to these guidelines [909]. The four principles of the Casley-Smith method for the treatment of lymphedema of the arm are skin care, manual lymphatic drainage, compression in the form of bandaging and/or garments, and exercise. Both benzopyrones and exercise will produce a continued reduction after the treatment course. They are particularly useful in a less compliant patient. It is stressed that the effect of patient compliance, particularly after treatment, makes a great difference to the ongoing success of the regime. A comparison between the efficacy of various current treatments and their cost shows that this combined and conservative method of treatment should be considered before recourse to pumps or surgery. The latter seldom achieve the results of decongestive lymphatic drainage, and, in the long term, they are more expensive. Certain preventive measures may be indicated following e.g., mastectomies. Prevention of the onset of lymphedema is of extreme importance. However, a return to as normal a lifestyle as possible by the patient is also essential. The earlier treatment begins after the onset of lymphedema, the better the prognosis for the patient. Lymphedema can and should be treated [910]. The Vodder technique differs in the use of adapted pressure and its application. The constant change in pressure optimizes results, moving fluid in the skin, increasing lymphomotoricity, and softening fibrosis, with the positive side effects of reducing pain and relaxing tense muscles. Another difference from other methods is the technique of stretching skin, not sliding it. Because of the fluid content in lymphedema, which is different from all other edemas, the combination of manual lymphatic drainage with compression treatment is the only solution for this pathology. Depending of its severity, each case requires individualized treatment. Phase 1 (intensive treatment) consists of daily treatment with up to two sessions per day for up to two hours. This phase is combined with special, individual skin care and remedial exercise. In phase 2, the goal of treatment is to maintain the results achieved in phase 1. The frequency of treatment is changed, but there is still the need for permanent, continued therapy. In phase 1, an average reduction of more than 40% of edema volume is achieved. In phase 2, the results are maintained and, with repetitions of phase 1, further improvement is possible. Thus, long-term results with permanent improvement are possible. Because of the complexity of the technique, no one can learn manual lymphatic drainage in one week. Students require a great deal of correction, and the technique must be checked constantly. To become a certified Vodder 188 Ciro Comparetto and Franco Borruto therapy, a four-week education program must be completed, and reviews must be attended every two years to maintain certification. The best education produces the best results for patients as long as patients are compliant. Therefore, the Vodder School also includes a patient education program as part of its curriculum [911]. Recent research has investigated the use of fibrin sealant in ALND performed for staging breast cancer. The majority of complications of axillary dissections in breast surgery stem from the two factors that influence the formation of seromas, hematomas, and lymphedema: the oozing of small transected vessels and the creation of a reservoir due to the removal of tissue. An effective surgical adhesive could improve hemostasis and tissue adherence, thereby decreasing the frequency of these postoperative problems [912]. Axillary reverse lymphatic mapping (ARLM) is a surgical technique that was first described in 2007 as a method to prevent lymphedema by mapping and preserving the lymphatic drainage of the arm during SLNB or ALND procedures for breast cancer. The ARLM procedure was feasible during ALND. Nevertheless, it was restricted by low identification rate of arm lymph nodes during SLNB. ARLM was beneficial for preventing lymphedema. However, this technique should be performed with caution given the possibility of crossover SLN-arm nodes and metastatic arm nodes. ARLM appeared to be unsuitable for patients with clinically positive breast cancer due to oncological safety concern [913]. Thus, several problems remain to be resolved in the practical application of this technique.The ARLM technique had several limitations that include a poor success rate for identification of arm lymph nodes and lymphatics. The occurrence of common lymphatic drainage pathways of the arm and the breast in a subset of patients also raises concerns regarding its oncological soundness [914]. Identification rates of arm nodes were insufficient using blue dye. Although this was improved using radioisotopes, radioisotopes alone do not permit visual mapping of arm lymphatics. Fluorescence imaging may be useful to improve the identification rate of arm nodes and lymphatics. On the other hand, the arm nodes may be involved with metastatic foci in patients with extensive ALN metastases. Moreover, the SLN draining the breast may be the same as the arm node draining the upper extremity in a minority of patients. These issues represent important drawbacks of the ARLM procedure. The success of ARLM in reducing lymphedema has not yet been determined. Further studies are needed before this can be accepted as a standard procedure in surgical management of breast cancer [915-919]. Mastectomy will continue to play a substantial role in the treatment of breast cancer, because many women either are not candidates for or do not desire to have breast conservation. Many patients treated with mastectomy will desire reconstruction, and many of these will be advised to receive adjuvant radiotherapy, which has been shown to increase overall survival in certain high-risk patients. There continues to be considerable controversy regarding the compatibility of radiation therapy and breast reconstruction Breast Surgery Complications 189 due to increased complications and decreased cosmetic outcome. These can be minimized by careful modern surgical and radiation techniques, and in most cases the result is acceptable, including for reconstructions with prosthetic implants as well as autogenous myocutaneous flaps [920]. Burns occur rarely after breast reconstruction, and results from loss of sensory and thermoregulatory skin functions. The ineffectiveness of skin thermoregulatory reflexes, caused by different surgical procedures, plays an essential role in the pathogenesis of burns to reconstructed breasts. Tissue expansion and radiotherapy are also essential etiological factors [921]. Although several studies have found obesity to increase the risk of postoperative morbidity in autologous breast reconstruction, there remains some controversy over the influence of obesity for muscleconserving abdominal flaps, including muscle-sparing TRAM, DIEP, and SIEA flaps. In comparison to free TRAM flaps, muscle-conserving abdominal flaps showed a lower pooled incidence of flap loss, fat necrosis, and abdominal bulge or hernia in obese patients. Obesity increases the risk of both flap-related and donor-site complications in breast reconstruction using muscle-sparing TRAM, DIEP, and SIEA flaps. In comparison to conventional TRAM flaps, however, muscle-conserving abdominal flaps may have an advantage in reducing the morbidity in obese patients [922]. The TRAM procedure has gained popularity over the last decades as an autogenous technique for breast reconstruction. Several outcome studies have demonstrated complications from this procedure. Women should be informed about the possible complications prior to surgery. Physical therapists can play an important role in rehabilitation and education for patients who are planning to undergo or who have undergone the TRAM procedure. It is important that physical therapists become well acquainted with the surgical procedure and treatment guidelines to effectively treat patients who have undergone the TRAM procedure, especially as this procedure increases in popularity. In addition, it is important that further research be conducted to substantiate the valuable clinical contribution that physical therapy has on successful recovery following the TRAM operation [923]. There is scant literature regarding a recently identified clinical entity termed “red breast syndrome.” Its clinical presentation has been described as a noninfectious, self-limited erythema of a postmastectomy breast reconstructed using ADM. Its incidence, risk factors, pathophysiology, clinical course, management, and long-term sequelae are largely unknown [924]. ADM have been used in breast surgery for a decade. They are widely used in implant-based breast reconstruction to provide coverage of the inferolateral aspects of the prosthesis. Numerous benefits have been reported with this approach including improved fold control, better support and control of the implant pocket with concomitant reduced risk of malposition, and improved lower pole expansion. Seroma, infection, mastectomy skin necrosis, and expander/implant loss are the most commonly reported complications with this approach, and the incidences vary widely among studies. Patient selection and adherence to established intraoperative 190 Ciro Comparetto and Franco Borruto technique principles related to ADM use are both critical to minimizing the risk of complications. ADM are also being used in aesthetic breast surgery, revision breast surgery, and NAC reconstruction, but clinical experience is limited [925]. In the studies evaluated, ADM-assisted breast reconstructions exhibited a higher likelihood of seroma, infection, and reconstructive failure than prosthetic-based breast reconstructions using traditional musculofascial flaps. ADM is associated with a lower rate of capsular contracture. A careful risk/benefit analysis should be performed when choosing to use ADM in implant-based breast reconstruction [926]. Because so many women have had breast augmentation mammoplasty, it is inevitable patients in the primary care settings will have complications related to the procedure or the type of implant. The most common complications include: 1) 2) 3) 4) 5) 6) 7) changes in breast sensation; capsular contracture; calcifications; mammography distortion or inaccuracies; gel-bleed; implant rupture or leakage; and possible systemic reactions involving the immune system. The double-bubble deformity is a widely recognized complication of breast augmentation, but there have been very few articles in the peer-reviewed literature devoted exclusively to this topic. The key to understanding the causes and correction of the double bubble lies in an appreciation of the anatomy of the inframammary fold. Correction of the deformity varies depending on whether or not patients had preexisting anatomical features predisposing them to development of a double bubble. A variety of surgical strategies, including use of a dual-plane pocket, form-stable shaped implants, capsulorrhaphy, pocket plane conversion, and use of ADM can play a role in prevention and treatment of the double-bubble deformity [927]. Recent worldwide media speculation that silicone gel-filled breast implants may be linked to an increased incidence of breast and other cancers, and connective tissue disease (particularly systemic sclerosis) has caused concern to the medical profession and public alike. Until carefully controlled studies have been performed to prove the safety of these implants, the FDA has restricted their use to research and breast reconstruction. Research has so far failed to prove a causative relationship between silicone implants and cancer or connective tissue disorders [928]. The silicone gel breast implant controversy has generated much confusion, making informed decision making for physicians and patients increasingly difficult [929]. The FDA’s decision to limit the use of silicone gel breast implants was surrounded by a great deal of misleading Breast Surgery Complications 191 information in the popular press [930]. Review of the literature suggests that silicone, a man-made polymer containing the element silicon, does not appear to fulfill the characteristics of an ideal synthetic soft-tissue substitute, although it may be the best substitute available. Silicone breast implants are associated with local inflammation and tissue fibrosis, with breast fibrous capsule contracture developing in 10-40% of the patients. There are no epidemiological data that establish a direct link between silicone and cancer or rheumatic disease. However, scleroderma appears to be over-represented among the published articles on patients with silicone breast implants and rheumatic disease. Autoantibodies of unclear significance may be found in 5-30% of women with silicone breast implants [931]. In 1992, the FDA announced that breast implants filled with silicone gel would be available only through controlled clinical studies despite the fact that they had been used for mammoplasty in millions of women around the world for more than 30 years. The safety of silicone gel breast implants had come into question after several reports on a possible association between the implants and subsequent development of connective tissue diseases. Risk assessment refers to the systematic, scientific characterization of potential adverse effects of human exposures to hazardous agents or activities. There now appears ample evidence from the scientific literature for the safety of these prostheses [932]. So, they were reintroduced in 2006, with a call for improved surveillance of clinical outcomes. There were possible associations with decreased risk for primary breast and endometrial cancers and increased risks for lung cancer, rheumatoid arthritis, Sjögren syndrome, and Raynaud syndrome. Evidence on breast implants and other outcomes either was limited or did not exist. The evidence was most frequently not specific to silicone gel implants, and studies were rarely adequately adjusted for potential confounders. The evidence remains inconclusive about any association between silicone gel implants and long-term health outcomes. Better evidence is needed from existing large studies, which can be reanalyzed to clarify the strength of associations between silicone gel implants and health outcomes [933]. In recent years, requests for breast implant surgery have occurred for several reasons. First, the number of diagnosed breast cancer cases has increased, and the number of reconstructive surgeries consequently has multiplied. Second, the number of patients who constantly try to achieve a better physical shape, corresponding in Western countries to the common image of prosperous and tonic breasts, has proliferated. These circumstances have led to an increasingly frequent need for more accurate and sophisticated imaging methods to study prosthetic breast implants and their integrity. Rupture of a breast implant is a recognized complication of augmentation mammoplasty and reconstructive breast surgery. Diagnostic imaging for the study of patients with suspected breast implant ruptures uses different techniques of radiological investigation such as mammography and ultrasonography, even if the current gold standard is MRI. In fact, proper cooperation and coordination between radiologists and dedicated plastic 192 Ciro Comparetto and Franco Borruto surgeons are fundamental for the proper management of patients and the complications they may experience [934]. Due to concerns over the extravasation of silicone gel within adjacent tissue and distant body sites, considerable attention has been given to the radiographic detection of mammary implant rupture. Mammography supplemented with ultrasonography constitutes the most cost-effective initial study, followed by MRI if these are equivocal. MRI is the most sensitive and specific study to evaluate breast implant rupture [935]. Despite numerous studies advocating ultrasonography and MRI in the evaluation of women with possible silicone breast implant rupture, an appropriate algorithm has not been published for the optimal use of these tests. In asymptomatic patients, the pretest rupture prevalence is 6.5%. If a screening ultrasonography shows no rupture, the probability of rupture drops to 2.2%. If ultrasonography shows rupture, the probability of true rupture increases to 37.8%. Removal of implants in this setting will result in a high probability of extracting normal implants. However, if MRI after the ultrasonography shows rupture, the probability of true rupture increases to 86%, which gives better assurance of removing true-ruptured implants. In “symptomatic” patients (i.e., breast asymmetry, capsular contracture) with implants ≤ ten years old, the prevalence of rupture is estimated at 31%. If ultrasonography shows no rupture, the probability of rupture drops to 16%. If ultrasonography shows rupture, the probability of true rupture is 79.7%, and this probability increases to 97.5% if a follow-up MRI also shows rupture. In symptomatic patients with implants more than ten years old, the prevalence of rupture is estimated at 64%. If ultrasonography shows rupture, the probability of true rupture increases to 94%, and no further diagnostic work-up is necessary. In an asymptomatic patient who is worried about the integrity of her implants, ultrasonography should be used as an initial diagnostic test because of its lower cost. If ultrasonography shows no rupture, no further work-up is necessary. If ultrasonography shows rupture, the low probability (37.8%) of true rupture requires a confirmatory test using MRI. In “symptomatic” patients, the high prevalence of rupture markedly raises the post-test probability of rupture for positive ultrasonography findings. Particularly in “symptomatic” patients with implants more than ten years old, the high post-test probability of rupture (94%) with a positive ultrasonography obviates the need for any further diagnostic testing. This diagnostic algorithm will assist plastic surgeons in counseling women who are worried about the integrity of their silicone breast implants [936]. Fibrous capsular contracture is by far the most common cause of unsatisfactory results following augmentation mammoplasty [937]. Capsular contracture is a common complication associated with the use of breast implants. Numerous RCT addressing the efficacy of textured surface breast implants in reducing capsular contracture have yielded non-uniform results. Only three of these studies found significantly lower rates of capsular contracture with the use of textured implants. However, when all seven studies Breast Surgery Complications 193 were pooled, the OR was found to be 0.19 [95% confidence interval (CI), 0.07-0.52], indicating a protective effect for surface texturing on the rate of capsular contracture. Submuscular placement was the only subgroup in which significance was not achieved. However, this subgroup consisted of a single study, which was dramatically underpowered. The results demonstrate the superiority of textured over smooth breast implants in decreasing the rate of capsular contracture [938]. The silicone granuloma was first described in the literature in 1964. During the course of clinical practice, the plastic surgeon will encounter a silicone granuloma in a breast augmentation or reconstruction patient. Although case series have been reported, there is no consensus regarding the treatment of silicone granulomas. This important clinical entity warrants investigative attention to better understand its clinical implications and prevention [939]. Capsular contracture frequently requires revision surgery. “Capsulectomy, site change, and implant exchange” has been referred to as the gold standard treatment of clinically significant contractures. However, the actual clinical evidence behind this algorithm remains elusive at best. There is limited clinical evidence behind the surgical management of capsular contracture. Site change and implant exchange are associated with reduced contracture recurrence rates and likely play a beneficial role in treating capsular contracture. The data on capsulectomy are less conclusive. ADM may be a useful adjunct but still requires long-term data [940-942]. Infection after breast implant surgery occurs in 1.1-2.5% of procedures performed for augmentation and up to 35% of procedures performed for reconstruction after mastectomy. Infection following breast implants is an uncommon event. This is somewhat surprising, since the human breast is not a sterile anatomical structure. The flora found in the breast are derived from the nipple ducts and closely resemble those of normal skin. Most infections result from skin organisms and occur in the immediate postoperative period, although infections can occasionally present after many years. These organisms, predominantly Staphylococcus epidermidis, may in some cases be responsible for firmness secondary to capsular contracture. Diagnosis of breast implant infection relies on the clinical presentation of breast pain, swelling, erythema, and drainage in conjunction with ultrasound-guided cultures of periprosthetic fluid. Treatment of the periprosthetic infection usually involves implant removal, but salvage by systemic antibiotics is sometimes possible. Atypical mycobacteria are very rarely the cause of infection, but can be extremely difficult to eradicate when involved. Toxic shock syndrome has been reported to occur following breast implants and is a lifethreatening problem requiring immediate removal of the implant. It may be significant that in some cases with effusion and infection occurring many months or years after implant placement, there has been a preceding event such as a laryngitis or flu-like illness. This suggests the possibility of a bacteremia being involved in the causation of the infection. If this were the case, then these patients should be handled in a fashion 194 Ciro Comparetto and Franco Borruto similar to those with prosthetic heart valves. Accordingly, it is advised that penicillin “V” be given beforehand when a patient with breast implants is to have any dental procedure. It must be stressed that there is no statistical or scientific proof at the present time that this is of any value. In conclusion, when dealing with these large foreign bodies, absolute sterility is essential, and excellent surgical technique to obviate hematoma and the occurrence of tissue ischemia is mandatory. Evidence of severe infection necessitates implant removal, but in less severe cases a trial of i.v. antibiotics is permissible. Having removed an implant, further insertion should be deferred, preferably for six months. If the new implant can be placed in a different plane, that is, submuscular, this is desirable. Exposed implants can be salvaged but this requires considerable judgment and one should be prepared for re-exposure or frank infection [943-946]. Silicone gel implants for breast augmentation and reconstruction have been in use since 1962. Significant local complications include capsular contracture, rupture, gel “bleed,” and spread of the implant material to RLN as well as histological findings of foreign body granulomas in the capsular tissue and in lymph nodes. Through MRI spectroscopy and atomic emission spectroscopy, silicon compounds were found in the blood of some women with silicone breast implants. Silicone and silica have also been found in liver. Well-publicized case reports have raised significant concerns regarding an association between implants and systemic disease. However, despite the availability of silicone implants for over 30 years, controlled epidemiological studies were not carried out until 1992. Currently available epidemiological data are extremely limited. In part, because the majority of implants were used after 1981, the incidence of long-term problems is not yet known [947]. Connective tissue disease occurring after cosmetic surgery with crystalline silica (silicone) injections or implants have been described in patients who have had silicone augmentation mammoplasty. This disorder which consists of autoimmunity-related complications, collectively known as autoimmune/inflammatory syndrome induced by adjuvant (ASIA), has been called “human adjuvant disease.” Previous data suggest that while some patients tend to develop postexposure autoimmune phenomena such as ASIA, other do not. However, thus far, no criteria for risk stratification were suggested. Therefore, a recommendation was made to avoid silicone implantation, e.g., individuals with previously diagnosed autoimmune disorders or with genetic preponderance for hyperactive immune system should not be considered as candidates for silicone implantation [948]. Although otherwise indistinguishable from other connective tissue disease, these patients may experience improvement or remission following implant removal. Current data suggest that the risk of disease after silicone breast augmentation is less than 1% [949, 950]. Silicone was originally regarded as inert in the human body. Silicone medical devices have been associated with various complications that may involve an immune reaction to silicone or a silicone organic Breast Surgery Complications 195 complex which can provoke a varied systemic autoimmune illness in patients who have had various foreign materials placed in the breast. Controversy exists as to which complications have a cause and effect relationship, and which represent coincidental findings. It is difficult to distinguish between non-specific local reactions and reactions that have an immunological basis. More than 2,000,000 women in the USA have had silicone breast implants inserted for reconstruction or augmentation mammoplasty: Since first reported in 1982, published anecdotal reports have appeared with increasing frequency of patients in whom autoimmune connective tissue diseases developed after mammary augmentation with silicone gel-filled elastomer envelope-type prostheses. Although scleroderma has been reported most often, other diagnoses have included SLE, rheumatoid arthritis, Sjögren’s syndrome, and mixed connective tissue disease. Other patients have ill-defined connective tissue-like illnesses. The occurrence of dermatomyositis and polymyositis after silicone breast implants appears to be infrequent [951]. Data on the reported cases do not in any way prove a causal relationship between breast implants and immune disease. Given the natural incidence of autoimmune diseases, we would expect a coincidental occurrence in the USA of more than 2,000 cases of autoimmune disease in women who had undergone breast implant surgery. Additional information must be obtained to resolve the question. The true incidence of autoimmune disease in patients with implants needs to be determined. A prospective registry of implant patients should be established and comprehensive retrospective information obtained on the implant patient population. Further experimental work is necessary on the bioreactivity of silicone. Patients with implants and autoimmune disease, once identified, must be carefully evaluated by physicians who are experienced in the treatment of autoimmune disease [952, 953]. Although silicone breast implants have been linked to various short-term complications, less is known about their long-range effects. Most attention has focused on connective tissue disorders, but the range of immunological disturbances observed in women with implants suggests that consideration also be given to other chronic diseases, including cancer. Recent controversy has arisen regarding the potential carcinogenesis of medical silicone-gel as used in breast implants. The greatest attention has focused on breast cancer, given clinical reports suggesting an association and observations that mammographic visualization is deterred by implants. Findings from epidemiological studies, however, actually suggest that breast cancer risk might be reduced among women with implants, although the biological mechanism remains undefined. In addition, most studies do not suggest that women with breast implants have more advanced breast cancer at diagnosis or a worse prognosis than those without implants. The majority of studies have focused on women who received implants for cosmetic reasons, with little previous investigation of women who received implants for breast reconstruction following cancer surgery. In terms of other cancers, animal as well as 196 Ciro Comparetto and Franco Borruto clinical data suggest potential risks of sarcomas and hematological cancers, including multiple myeloma. A review of the pertinent literature shows that although rodents do demonstrate the development of sarcomas to any inert material, including silicone-gel, the phenomenon is species specific. The risk of these cancers has not yet been adequately addressed by epidemiological studies, although several ongoing studies should provide insights. It will be important for studies to consider effects of other lifestyle factors as well as to analyze relationships according to duration of implantation, a demonstrated determinant of implant deterioration. In addition, consideration should be given to type of implant, including implants with polyurethane foam covers, which can leak toluene diamine, a demonstrated carcinogen in animals [954, 955]. So, although surgical implantation of breast prostheses has become increasingly popular while the incidence of breast cancer is increasing each year, there has been no definitive consensus regarding the casual relationship between augmentation mammoplasty and breast cancer incidence, detection, treatment, mortality, and survival. The published evidence, including epidemiological studies and case reports, states that there is no breast cancer risk in prior augmented women. Moreover, there is also no significant difference in frequency, stage, or mean tumor size between augmented and non-augmented women [956]. The challenges of managing breast cancer in women with augmented breasts include screening, diagnosis, oncological and revisional surgery, and surveillance. In addition, women with augmented breasts frequently have greater expectations of cosmetic outcomes. More breast clinicians will be affected by these challenges as augmentation grows in popularity and women with implants reach the age range in which they are at higher risk of developing breast cancer. In the USA, more than two million women have undergone augmentation, making this the second most commonly performed cosmetic procedure. With a lifetime risk of developing breast cancer of one in eight, it is projected that more than 50,000 women who undergo augmentation each year in the USA will develop breast cancer at some point in their lives. Management of breast cancer in previously augmented breasts presents a unique range of challenges. Patients can be reassured that the presence of an implant does not increase the risk of breast cancer developing or affect the prognosis if breast cancer does develop. Clinical judgement is made balancing surgical and oncological principles to provide the best cosmetic outcome [957]. There are increasing concerns about a possible association, without evidence of causation, between kinase-negative anaplastic large cell lymphoma (ALCL), a rare nonHodgkin’s T-cell lymphoma, and breast implants: 1) there is a positive association between breast implants and ALCL development, but likely under-recognition of the true number of cases; Breast Surgery Complications 197 2) a recurrent, clinically evident seroma occurring six months or more after breast implantation should be aspirated and sent for cytologic analysis; 3) kinase-negative ALCL that develops around breast implants is a clinically indolent disease with a favorable prognosis that is distinct from systemic kinasenegative ALCL; 4) management should consist of removal of the involved implant and capsule, which is likely to prevent recurrence, and evaluation for other sites of disease; and 5) adjuvant radiation or chemotherapy should not be offered to women with capsule-confined disease. Little agreement, however, was found regarding etiologic risk factors for implantassociated ALCL. Breast-associated ALCL occurred in women with and without implants. No prospective epidemiological study has linked implants and ALCL. However, a single case-control study in Dutch women reported increased odds of association between ALCL and implants, and an estimated frequency of one in one million women with and without breast implants. An association, without evidence of causation, was reported between breast implants and ALCL. Breast-associated ALCL occurred rarely in women with and without breast implants and had a primarily indolent clinical course, which may provoke a revision of the WHO nomenclature for lymphoma: however, aggressive clinical behavior was also reported. The cases of ALCL were not confined to a specific type of implant. Public awareness has increased following a safety communication warning of the association of breast implant-associated ALCL by the FDA in 2011. Difficulty with determining an accurate assessment of risk, including diagnosis, or standardized treatment regimen has led surgeons to commonly omit preoperative discussion of this rare and frequently misunderstood cancer. Risk disclosure is a form of respect for patient autonomy, and informed consent has positive practical and moral consequences for the practice of plastic surgery. Breast implant-associated ALCL should be included during preoperative counseling on the risks of breast implantation when obtaining informed consent. Pertinent aspects of decision-making include disease awareness, presenting symptoms, and resources for concerned patients. Education of health care professionals and provision of patient-focused materials ensures effectiveness of the informed consent process. Substantial further research is needed to improve our understanding of the epidemiology, clinical aspects, and biology of this disease [958-964]. Although many patients who undergo reduction mammoplasty are obese, reports on whether obesity is a risk factor for postoperative complications have been conflicting. A meta-analysis indicated that the risk of surgical complications and tissue necrosis after reduction mammoplasty is higher in obese patients than in non-obese patients and that 198 Ciro Comparetto and Franco Borruto the risk gradually increases with an increase in the severity of obesity. The findings of this study could form a basis for preoperative patient education, surgical method selection, and determination of the extent of postoperative care [965]. Owing to the relatively short time endoscopic-assisted plastic surgery procedures have been done, there are few published reports of complications. The complications associated with endoscopic techniques are similar to those with open techniques. It is clear, however, that a subset of complications specific to endoscopic procedures exists. As endoscopic techniques and instrumentation are further developed, and as surgeons move higher up on the “learning curve,” these complications should be reduced [966, 967]. Over the last few decades, the psychosocial problems following mastectomy have become well recognized and surgical treatment for breast cancer has become less mutilating. For women with small cancers, a number of equally effective treatments are available and involving the patient in the choice of treatment appears to reduce morbidity [968]. Therefore, in recent years, doubt has been shed on the necessity of mastectomy for women with early-stage breast cancer. Apart from purely medical studies comparing radical mastectomy to less intruding surgical treatment, a number of studies have been published investigating the impact of breast-conserving treatment versus mastectomy on quality of life. With respect to medical issues (treatment modality, stage of disease), methodological issues (design, measurement moment, and sample size), and results (psychological discomfort, changes in life patterns, and fears and concerns), there is no solid proof of a better psychological adjustment after breast-conserving treatment and there are no substantial differences between the different treatment modalities in changes of life patterns and fears and concerns. However, the results with respect to body image and sexual functioning favor the use of breast-conserving treatment [969]. Breast cancer has been widely studied with respect to its psychological impact because it is a disease which threatens an organ that is intimately associated with self-image, self-esteem, sexuality, femininity, and reproductive and nurturing capacity. Breast cancer is a significant stress for any woman: however, women vary widely in their response to diagnosis and treatment. The following issues: social, psychological, and medical variables contribute to the psychological response of women in the pre- and postsurgical treatment period [970]. From the available evidence, it is clear that mastectomy is associated with a substantial psychological and psychiatric morbidity. To date, there is no convincing evidence that counseling can prevent this morbidity, but monitoring of women’s psychological adjustment can lead to early detection and effective treatment of their problem. The use of immediate or delayed implantation or reconstruction appears to reduce the psychiatric morbidity in those women who are particularly concerned about their appearance at the time of surgery. Psychiatric morbidity is further increased when adjuvant chemotherapy is used and when treatment results in persistent arm pain and swelling. A shorter course of adjuvant chemotherapy and reduction of surgery within the Breast Surgery Complications 199 axilla could reduce psychiatric morbidity. The role of radiotherapy is still unclear, but in some studies a link has been found between the amount of radiotherapy given, adverse effects, and psychiatric morbidity. In women undergoing breast conservation, the reduction in body image problems is offset by greater anxiety about recurrence and depression caused by radiotherapy. Exploring and allowing choice when a patient has a strong preference for breast conservation or mastectomy appears to reduce morbidity. However, attention still needs to be paid to the early recognition and treatment of psychological problems in patients with breast cancer [971]. Young women undergoing treatment for breast cancer remain understudied despite unique needs. Psychoeducational interventions help to relieve symptoms and emotional distress during treatment, but effects do not appear to persist over the longer term. In the clinical context, the performance of prognostic risk prediction models in this population is poor. Surgical decision-making is often driven by fear of recurrence and body image rather than prognosis, and decision aids may help young women to synthesize information to preserve their role in the treatment process. First, shared decision-making, second, balancing body image, fear of recurrence, and recommended treatment, and third, palliative care for metastasis are essential research priorities for the clinical setting. In the larger social context, unique family/partner dynamics as well as financial and insurance concerns warrant particular attention in this population [972]. Cancer is a family affair. Clinical work and research studies have shown that cancer does indeed invade the entire family, and that family members, especially spouses, are often highly distressed individuals. The family in general and the spouse in particular cannot, therefore, be looked on as natural supporters for cancer patients, but rather as a system that is itself in need of help and support [973]. Breast cancer affects a woman’s body image and feelings of sexuality. Little is known about the perceptions of spouses to the sensitive topics of sexuality and body image. A qualitative inquiry was undertaken using in-depth interviews. A diagnosis of breast cancer brought some relationships closer. Mastectomy by disturbing body image did obliterate sexual relationships for a significant period of time. Women often felt (wrongly) that their partner would be repulsed by changes. More support in relation to sexuality and body image could improve relationships by identifying and clarifying perceptions, and therefore the quality of life [974]. A growing body of evidence suggests that sexual dysfunction may be among the more common and distressing symptoms experienced by breast cancer survivors. A review of the literature suggests a wide range of rates for the prevalence of sexual problems in breast cancer survivors. Factors that may affect prevalence rates include the methods used to determine prevalence and the demographic and medical characteristics of the patients studied. With regard to treatment effects, evidence suggests that breast cancer patients who undergo chemotherapy are at high risk for sexual dysfunction after treatment. In contrast, there is little evidence of a link between type of 200 Ciro Comparetto and Franco Borruto surgical treatment (e.g., lumpectomy vs mastectomy) or treatment with TMX and sexual functioning outcomes. A growing body of evidence suggests that sexual problems can be a long-term side effect of breast cancer treatment. Oncology professionals should initiate communication about sexual difficulties, perform comprehensive assessments, and educate and counsel patients about the management of these difficulties [975, 976]. An unexpected statistically significant relationship between cosmetic breast implants and suicide has been found in some epidemiological investigations completed in the last several years. Across the studies, the suicide rate of women who received cosmetic breast implants is approximately two- to three-fold the expected rate based on estimates of the general population. Although the first study of this issue suggested that the rate of suicide among women with breast implants was greater than that of women who underwent other forms of cosmetic surgery, the largest and most recent investigation in this area found no difference in the rate of suicide between these two groups of women. Over the last decades, of the large number of epidemiological studies on various longterm adverse health outcomes among implant recipients, this excess of suicide is the only serious adverse effect supported by credible research. But an association of suicide with cosmetic breast implants based on comparisons with the general population does not by itself support a cause-and-effect relationship regarding the role of implants. The higherthan-expected suicide rate among women with cosmetic breast implants warrants further research. Etiological-epidemiological research is urgently needed to evaluate whether the association between breast implants and suicide is spurious (e.g., reflecting increased prevalence of underlying psychopathology and other risk factors for suicide in women seeking implants) or reflects an actual role for implants in suicide. As a first step, sound epidemiological nested case-control studies are required to characterize the preimplant psychiatric history of women with implants who commit suicide compared with women with implants from the same cohort who do not commit suicide. Second, large-scale retrospective cohort studies are needed to compare suicide rates among women with implants versus those among women without implants matched on preimplant diagnoses of psychiatric disorders, year of diagnosis, year of birth, family history of psychiatric admissions, and other relevant factors that influence suicide risk. This type of large-scale complex epidemiological research may be difficult to complete in countries with restrictive privacy laws and widespread litigation, but only after such appropriate etiological research is completed can any credible cause-effect inference be made regarding the role, if any, of cosmetic breast implants in suicide. In the absence of additional information on the relationship, women interested in breast augmentation who present with a history of psychopathology or those who are suspected by the plastic surgeon of having some form of psychopathology should undergo a mental health consultation before surgery [977-979]. Chapter XII Anesthesiological Issues Pain is the most distressing symptom in patients with breast cancer and can occur at all stages of the disease due to the cancer per se as well as due to various diagnostic and treatment modalities. Although the genesis of the pain is multifactorial, sectioning of the intercostobrachial nerve and related injury of the intercostal nerves and other nerves in the region are the nerve lesions diagnosed more often. Persistent pain after breast cancer surgery is increasingly recognized as a potential problem facing a sizeable subset of the millions of women who undergo surgery as part of their treatment of breast cancer. Importantly, an increasing number of studies suggest that individual variation in psychosocial factors such as catastrophizing, anxiety, depression, somatization, and sleep quality play an important role in shaping an individual’s risk of developing persistent pain after breast cancer surgery [980]. A proper pain assessment helps in identification of pain syndromes and guides in formulating analgesic strategies. Primary therapies of breast cancer like surgery, chemotherapy, and radiotherapy for bony metastases can cause substantial pain relief. However, multimodal analgesic approaches incorporating pharmacological, interventional, as well as non-conventional techniques should be employed prior to, in conjunction with, and after primary therapies of breast cancer. Patients post mastectomy and breast reconstruction can suffer from acute nociceptive pain and chronic neuropathic pain syndromes. The prevalence of chronic neuropathic pain following breast cancer surgery may exceed 50% by current estimates, and with the increase in life expectancy of these patients, providing adequate pain relief is of paramount importance to improve their quality of life [981]. Several preventative measures to control acute postoperative pain and chronic pain states such as postmastectomy pain and phantom pain have been tried [982]. The approach to patients undergoing surgery for breast cancer requires pre- and postoperative follow-up by a multidisciplinary team. This approach can provide a rational choice of surgical 202 Ciro Comparetto and Franco Borruto technique, identify patients with risk factors, minimize or eliminate risk factors whenever possible, diagnose beforehand the postmastectomy pain syndrome, and provide adequate treatment to improve the quality of life for this specific patient population [983]. Postoperative pain management following breast surgery has traditionally involved i.v. and oral narcotics. However, pain control is not always adequately achieved through these means and may cause unwanted side effects, including headache, nausea, vomiting, constipation, altered mental status, sleep disturbance, and respiratory depression [984]. Regional analgesia, opioids, and several oral analgesics are commonly used for the treatment of acute pain after breast cancer surgery. While all of these treatments can suppress the acute postsurgical pain, there is growing evidence that suggests that the postsurgical comorbidity will differ in accordance with the type of analgesic used during the surgery. A considerable number of clinical studies have been performed to investigate the relationship between the acute analgesic regimen and common comorbidities, including inadequate quality of recovery after the surgery, persistent postsurgical pain, and cancer recurrence. Previous studies have shown that the choice of the analgesic modality does affect the postsurgical comorbidity. In general, the use of regional analgesics has a beneficial effect on the occurrence of comorbidity. In order to determine the best analgesic choice after breast cancer surgery, prospective studies that are based on a clear definition of the comorbidity state will need to be undertaken in the future. Alternative forms of pain control have been used successfully in other surgical fields but have been utilized only recently in breast surgery [985]. Neural blockade can be useful for the identification of nerves involved in neuropathic pain syndromes or to be used as a treatment in its own right [986, 987]. Paravertebral blocks are becoming increasingly popular, especially as an anesthetic adjunct for breast procedures. New reports suggest additional reasons for adding this block to the anesthetic armamentarium. Recent studies demonstrate a benefit from preoperative placement of a paravertebral block, not only in reducing acute postoperative pain, but also statistically significant reductions in the percentage of patients that develop chronic postsurgical pain one year after surgery. Another study found that the breast-cancer recurrence rate at 36 months after surgery was lower in the paravertebral group compared with the general anesthesia-only group of patients. Paravertebral blocks are a well-established option to provide anesthesia and postoperative analgesia during breast surgery. Recent studies suggest additional benefits to this procedure. Not only is acute pain better controlled, but the development of chronic mastectomy pain syndrome and recurrence of cancer may be reduced by preoperative placement of paravertebral block. These studies provide additional reasons why this block should be considered as part of the anesthetic for breast surgery [988]. It is well established that thoracic paravertebral block with or without general anesthesia provides better postoperative analgesia and reduces the risk of nausea and vomiting after breast surgery as well as the Anesthesiological Issues 203 incidence of chronic pain. Paravertebral block improves the quality of recovery after breast cancer surgery and provides the patient with the option of ambulatory discharge [989]. Propofol-paravertebral anesthesia is a unique combination of paravertebral nerve blocks and propofol that regulates the cellular microenvironment during surgical period. Growing evidence points to its ability to attenuate perioperative immune-suppression of cancers. Abundant studies show that cancer patients who undergo perioperative propofol-paravertebral anesthesia exhibit less recurrence as well as metastasis. Over the last decades, increasing concerns have been put on the promotional role of propofolparavertebral anesthesia in the prognosis of breast cancer patients. Among them, propofol-paravertebral anesthesia participates in several bioprocesses in the development of breast cancer, including inhibiting hypoxia-inducible factor (HIF) activity, elevating serum concentration of nitric oxide index (NOx), depression of the neuroepithelial cell transforming gene 1 (NET1) signal pathway, blocking the nuclear factor kappa B (NFκB) pathway following a decreased expression of matrix metalloproteinase (MMP), increasing natural killer (NK) cytotoxicity, and affecting transforming growth factor (TGF)-β-targeted ras and HER2/neu gene pathways. This will provide an alteration pattern of surgical anesthesia technique in breast cancer patients with poor prognosis [990-992]. Short-term postsurgical recovery is complicated by many factors, including imbalanced inflammatory and immune response, acute pain associated with functional impairment, and chronic postmastectomy pain. Opioids, most common drugs used for treatment of cancer pain, are immune-suppressive, and therefore, they might directly and/or indirectly influence long-term cancer recurrence. Moreover, they also produce endocrinopathy, which consists primarily of hypothalamic-pituitary-gonadal axis or hypothalamic-pituitary-adrenal axis dysfunction. The interindividual variability in both chronic postmastectomy pain and opioid response is believed to be largely underlined by genetic variability in the gene locus for μ-opioid receptor (OPRM1) that modulates opioid pharmacodynamics. For this reason, OPRM1 genotype may play a key role both in short-term postmastectomy outcome and in long-term follow-up, becoming a new biomarker for breast cancer recurrence in patients suffering from chronic postmastectomy pain managed by opioid therapy. Hence, OPRM1 might be used in near future to customize the opioid therapy, avoiding not only opioid side-effects but also the disease progression. Therefore, a personalized pain treatment strategy might be useful to both manage pain and control cancer disease progression [993]. The effect of pharmacological analgesic modalities for breast cancer surgery interventions is varied [non-steroidal anti-inflammatory drugs (NSAIDS), opioids, anticonvulsants, ketamine, and lidocaine]. Likewise, data from high-quality RCT on wound infiltration (including liposome encapsulated) and infusion of local anesthetic are minimal and conflicting. Conversely, abundant evidence demonstrates paravertebral blocks and thoracic epidural 204 Ciro Comparetto and Franco Borruto infusions provide effective analgesia and minimize opioid requirements, while decreasing opioid-related side effects in the immediate postoperative period. Other techniques with promising – but extremely limited – data include cervical epidural infusion, brachial plexus, interfascial plane, and interpleural blocks. In conclusion, procedural interventions involving regional blocks are more conclusively effective than pharmacological modalities in providing analgesia to patients following surgery for breast cancer [994]. Breast surgery performed under general anesthesia is associated with a high incidence of postoperative nausea and vomiting. Between 60-80% of patients undergoing mastectomy (with ALND) experience postoperative nausea and vomiting. First, the risk factors related to patient characteristics, surgical procedure, anesthetic technique, and postoperative care can be reduced. More specifically, the use of propofol-based anesthesia can reduce the incidence of postoperative nausea and vomiting. Secondly, a wide range of prophylactic antiemetics, including butyrophenones (droperidol), benzamides (metoclopramide), glucocorticoids (dexamethasone), clonidine, a small dose of propofol, and serotonin receptor (SR) antagonists (ondansetron, granisetron, tropisetron, dolasetron, ramosetron, and palonosetron), are available for preventing postoperative nausea and vomiting after breast surgery. Traditional antiemetics (droperidol and metoclopramide) are frequently used for the prevention of postoperative nausea and vomiting during the first 24 hours after anesthesia. The available nontraditional antiemetics that have been shown to be effective for prophylaxis against postoperative nausea and vomiting are dexamethasone, clonidine, propofol, and supplemental oxygen. Antiserotonins (ondansetron, granisetron, tropisetron, dolasetron, and ramosetron) are highly effective for preventing postoperative nausea and vomiting for 24 hours postoperatively, compared with traditional antiemetics. Ramosetron is effective for the long-term (up to 48 hours) prevention of postoperative nausea and vomiting. Better results can be obtained by combining antiemetics, because they have different sites of action. Combination antiemetic therapy is often effective for preventing postoperative nausea and vomiting after breast surgery. Thirdly, antiemetic therapy combined with granisetron and droperidol or dexamethasone, and a multimodal management strategy which includes a package consisting of dexamethasone, total i.v. anesthesia with propofol, and ondansetron are highly effective in preventing postoperative nausea and vomiting. Combinations of an antiserotonin (granisetron or dolasetron) and droperidol or dexamethasone are more effective than monotherapy with antiserotonins. Fourth, electroacupoint stimulation at the P6 point (Nei-Guwan) as a nonpharmacologic therapy is as effective as ondansetron for preventing postoperative nausea and vomiting. Overall, these pharmacological and non-pharmacological approaches reduce the incidence of postoperative nausea and vomiting following breast surgery. Most of the published trials indicate improved prophylaxis of postoperative nausea and Anesthesiological Issues 205 vomiting following breast surgery by avoiding risk factors, and by using effective antiemetic agents in women scheduled for mastectomy (with ALND). The clinician must weigh the benefits of using pharmacological and non-pharmacological approaches for postoperative nausea and vomiting against the risk of occurrence of adverse events. Knowledge of the risk factors for postoperative nausea and vomiting, antiemetics, and a non-pharmacological approach are needed for the management of postoperative nausea and vomiting in women undergoing breast cancer surgery [995, 996]. The introduction of enhanced recovery after surgery protocols has revolutionized pre- and postoperative care. To date, however, the principles of enhanced recovery have not been applied specifically to patients undergoing breast surgery. Use of the thoracic block, from both analgesic and anesthetic viewpoints, is well supported by evidence and should be encouraged. Trials specific to breast surgery regarding aspects such as perioperative fasting, preanesthetic medication, prevention of hypothermia, and postdischarge support are scarce, and evidence was extrapolated from non-breast trials. Trials on postoperative analgesia and prevention of postoperative nausea and vomiting in breast surgery are generally of small numbers. In addition, there is heterogeneity between studies. The principles of enhanced recovery can be adopted in breast surgery [997]. Chapter XIII Conclusion The outcome of breast cancer surgery, with respect to cosmetic results, loco-regional control, and prognostic information from nodal staging, may vary substantially. Optimal breast cancer care starts with a proper surgical act, which can only be performed when optimal imaging and preoperative diagnosis are available. Next, on the basis of all perioperative findings, the right surgical procedure should be indicated after multidisciplinary consultation and discussion, keeping the objective of the final outcome in mind. The surgical act itself is best performed by an experienced surgeon who has maintained his experience after sufficient training. The outcome of the different procedures can be measured according to simple criteria and prospective registration [998]. Many studies have demonstrated gaps in healthcare quality for all medical and surgical specialties, including breast surgical care. How to optimally measure and improve quality has generated debate at the local, state, and national level. Attempts to judge medical performance by private companies using non-risk-adjusted administrative databases may not be accurate and may unfairly penalize surgical care. Breast surgeons and their professional organizations need to take ownership of quality measure programs because others will surely do so if we do not. Participation in one or more of these programs is beneficial because peer performance comparison allows identification of potential areas for individual or institutional improvement and demonstrates the commitment of breast surgeons to quality improvement. This commitment may gain even greater importance if trends continue toward performance-based physician payment, patient steerage, licensure, and board certification [999]. Quality assurance is the process by which quality care can be assessed. The general principles include setting a standard with the aim of achieving particular outcomes, followed by the evaluation of parameters that allow for quality assessment. Loco-regional and survival outcomes are the major parameters but require years to evaluate and have other limitations. Other parameters 208 Ciro Comparetto and Franco Borruto therefore may assist in evaluation, such as the availability of the structures and processes required to achieve desired outcomes. Unlike chemotherapy and radiotherapy, the quality of surgery is difficult to quantify, yet it is central to the issue of loco-regional control and survival. In breast cancer surgery, quality control starts at the diagnostic service, from referral by the family practitioner to the appropriate triage of patients thereby preventing diagnostic delays. The surgical oncologist is pivotal in the multidisciplinary input necessary with both radiologists and pathologists in achieving the correct preoperative diagnoses of symptomatic and screen detected lesions as specified by many of the guidelines. Quality control of the operative surgery addresses issues such as training, volume, and life audit of the surgeon. Standardization of operative technique, pathology reporting with emphasis on specimen orientation and margins, and management of the axilla and how it impacts on adjuvant treatment are other important issues. More recently, the availability of breast reconstruction services and the development of the oncoplastic surgeon is becoming an important quality issue. Finally, the quality of the follow-up process provides the tools to assess the outcome of both the patient and the service [1000]. References [1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] Marchant, DJ. Role of the obstetrician/gynecologist in the management of breast disease. Obstet Gynecol Clin North Am, 1994 21(3), 421-431. Marchant, DJ. Diagnosis of breast disease and the role of the gynecologist. Curr Opin Obstet Gynecol, 1993 5(1), 67-72. Barber, RK. The gynecologist and breast disease. Eur J Gynaecol Oncol, 1992 13(1), 5-16. Fiorica, JV. Breast disease. Curr Opin Obstet Gynecol, 1992 4(6), 897-903. Mitchell, GW Jr. The gynecologist and breast disease. Clin Obstet Gynecol, 1977 20(4), 865-880. Bilous, M; Brennan, M; French, J; Boyages, J. Making sense of breast pathology. Aust Fam Physician, 2005 34(7), 581-586. Bradley, AL; Sharp, KW. Breast disease. Med Clin North Am, 1995 79(6), 14431455. Masood, S; Edwards, PD; Arnold, MJ. Breast health. Challenges and promises. J Fla Med Assoc, 1996 83(7), 459-465. Folk, FA. Breast surgery. Surg Clin North Am, 1977 57(6), 1173-1184. Isaacs, G. Breast shaping procedures, abdominoplasty, and thighplasty in Australia. Clin Plast Surg, 1984 11(3), 525-548. Davis, JE. Major ambulatory surgery of the general surgical patient. Management of breast disease and hernias of the abdominal wall. Surg Clin North Am, 1987 67(4), 733-760. Fisher, B. A commentary on the role of the surgeon in primary breast cancer. Breast Cancer Res Treat, 1981 1(1), 17-26. Teshome, M; Kuerer, HM. Training of breast surgical oncologists. Chin Clin Oncol, 2016 5(3), 43. 210 [14] [15] [16] [17] [18] [19] [20] [21] [22] [23] [24] [25] [26] [27] [28] [29] [30] References Throckmorton, A; VanderWalde, L; Brackett, C; Dominici, L; Eisenhauer, T; Johnson, N; Kong, A; Ludwig, K; O’Neill, J; Pugliese, M; Teller, P; Sarantou, T. The Ethics of Breast Surgery. Ann Surg Oncol, 2015 22(10), 3191-3196. Shuster, TD; Girshovich, L; Whitney, TM; Hughes, KS. Multidisciplinary care for patients with breast cancer. Surg Clin North Am, 2000 80(2), 505-533. Lyman, GH; Baker, J; Geradts, J; Horton, J; Kimmick, G; Peppercorn, J; Pruitt, S; Scheri, RP; Hwang, ES. Multidisciplinary care of patients with early-stage breast cancer. Surg Oncol Clin N Am, 2013 22(2), 299-317. Franceschini, G; Di Leone, A; Masetti, R. The Breast Unit Update on advantages and the open issues. Ann Ital Chir, 2014 85(5), 407-412. Taran, FA; Eggemann, H. Breast Units in Europe – Certification in 9 European Countries 9 Years after the European Society of Mastology Position Paper. Breast Care (Basel), 2009 4(4), 219-222. Silverstein, MJ. The Van Nuys Breast Center: the first free-standing multidisciplinary breast center. Surg Oncol Clin N Am, 2000 9(2), 159-175. Roux, S; Logan-Young, W. Private practice interdisciplinary breast centers: their rationale and impact on patients, physicians, and the health care industry: a bicoastal perspective. Surg Oncol Clin N Am, 2000 9(2), 177-198. Kolb, GR. Disease management is the future: breast cancer is the model. Surg Oncol Clin N Am, 2000 9(2), 217-232. Lee, CZ. Oncopolitical issues: obstacles and options for success in a comprehensive breast center. Surg Oncol Clin N Am, 2000 9(2), 279-294. Ibarra, JA. The pathologist in breast cancer: contemporary issues in the interdisciplinary approach. Surg Oncol Clin N Am, 2000 9(2), 295-317. Pockaj, BA; Gray, RJ. Current surgery for breast cancer. Future Oncol, 2009 5(4), 465-479. Slomiany, BA; Chagpar, AB. The surgical management of breast cancer. J Ky Med Assoc, 2009 107(10), 391-395. Tripathy, D. Multidisciplinary care for breast cancer: barriers and solutions. Breast J, 2003 9(1), 60-63. Truppman, ES; Schwartz, BM. Aesthetic breast surgery. J Fla Med Assoc, 1989 76(7), 609-612. Kissin, MW. The patron saints of breast disease. Aust N Z J Surg, 1991 61(6), 452-458. Pon, L; Amatruda, JF. Breast cancer between faith and medicine: the Peres Maldonado ex-voto. Med Humanit, 2010 36(2), 112-114. Vaidya, JS. Locally advanced breast cancer in a 15th century painting in Milan. Breast, 2007 16(1), 102-103. References [31] [32] [33] [34] [35] [36] [37] [38] [39] [40] [41] [42] [43] [44] [45] [46] [47] 211 Retief, FP; Cilliers, L. Breast cancer in antiquity. S Afr Med J, 2011 101(8), 513515. Sakorafas, GH; Safioleas, M. Breast cancer surgery: an historical narrative. Part I. From prehistoric times to Renaissance. Eur J Cancer Care (Engl), 2009 18(6), 530-544. Ellis, H. Amputation of the breast. J Perioper Pract, 2015 25(1-2), 27-28. Meyer, KK; Beck, WC. Mastectomy performed by Lawrence Heister in the eighteenth century. Surg Gynecol Obstet, 1984 159(4), 391-394. Androutsos, G; Karamanou, M. Bernard Peyrilhe (1737-1804) and the first experimental transmission of cancer. J BUON, 2009 14(4), 731-733. Sakorafas, GH; Safioleas, M. Breast cancer surgery: an historical narrative. Part II. 18th and 19th centuries. Eur J Cancer Care (Engl), 2010 19(1), 6-29. Izuo, M. Medical history: Seishu Hanaoka and his success in breast cancer surgery under general anesthesia two hundred years ago. Breast Cancer, 2004 11(4), 319-324. Sarhadi, NS; Shaw-Dunn, J; Soutar, DS. Nerve supply of the breast with special reference to the nipple and areola: Sir Astley Cooper revisited. Clin Anat, 1997 10(4), 283-288. Macmahon, CE; Cahill, JL. The evolution of the concept of the use of surgical castration in the palliation of breast cancer in pre-menopausal females. Ann Surg, 1976 184(6), 713-716. Egan, RL. Evolution of the team approach in breast cancer. Cancer, 1975 36(5), 1815-1822. Löwy, I. Breast cancer and the “materiality of risk”: the rise of morphological prediction. Bull Hist Med, 2007 81(1), 241-266. Paneth, N; Susser, E; Susser, M. Origins and early development of the casecontrol study: Part 2, The case-control study from Lane-Claypon to 1950. Soz Praventivmed, 2002 47(6), 359-365. de Moulin, D. Historical notes on breast cancer, with emphasis on the Netherlands. I. Pathological and therapeutic concepts in the seventeenth century. Neth J Surg, 1980 32(4), 129-134. Bilynskyj, BT. The breast cancer treatment as a marker of progress in oncology. Exp Oncol, 2010 32(3), 190-194. Plesca, M; Bordea, C; El Houcheimi, B; Ichim, E; Blidaru, A. Evolution of radical mastectomy for breast cancer. J Med Life, 2016 9(2), 183-186. Crile, G Jr. Breast cancer. A personal perspective. Surg Clin North Am, 1984 64(6), 1145-1149. Collins, JP. Mastectomy with tears: breast cancer surgery in the early nineteenth century. ANZ J Surg, 2016 86(9), 720-724. 212 [48] [49] [50] [51] [52] [53] [54] [55] [56] [57] [58] [59] [60] [61] [62] [63] [64] References Halsted, CP; Benson, JR; Jatoi, I. A historical account of breast cancer surgery: beware of local recurrence but be not radical. Future Oncol, 2014 10(9), 16491657. Beinfield, H; Beinfield, MS. Revisiting accepted wisdom in the management of breast cancer. Altern Ther Health Med, 1997 3(5), 35-53. Ghossain, A; Ghossain, MA. History of mastectomy before and after Halsted. J Med Liban, 2009 57(2), 65-71. Suami, H; Pan, WR; Taylor, GI. Historical review of breast lymphatic studies. Clin Anat, 2009 22(5), 531-536. Sakorafas, GH; Safioleas, M. Breast cancer surgery: an historical narrative. Part III. From the sunset of the 19th to the dawn of the 21st century. Eur J Cancer Care (Engl), 2010 19(2), 145-166. Newmark, JJ. “No ordinary meeting”: Robert McWhirter and the decline of radical mastectomy. J R Coll Physicians Edinb, 2016 46(1), 43-48. Lesnick, G. Modern surgical treatment of breast cancer. Jpn J Surg, 1985 15(6), 420-426. Becker, S. A historic and scientific review of breast cancer: The next global healthcare challenge. Int J Gynaecol Obstet, 2015 131 Suppl 1, S36-S39. Robinson, JO. Treatment of breast cancer through the ages. Am J Surg, 1986 151(3), 317-333. Fisher, B. Role of science in the treatment of breast cancer when tumor multicentricity is present. J Natl Cancer Inst, 2011 103(17), 1292-1298. Aronowitz, RA. Do not delay: breast cancer and time, 1900-1970. Milbank Q, 2001 79(3), 355-386. Fisher, B. From Halsted to prevention and beyond: advances in the management of breast cancer during the twentieth century. Eur J Cancer, 1999 35(14), 19631973. Zurrida, S; Costa, A; Luini, A; Galimberti, V; Sacchini, V; Intra, M. The Veronesi quadrantectomy: an established procedure for the conservative treatment of early breast cancer. Int J Surg Investig, 2001 2(6), 423-431. Veronesi, U; Zurrida, S. Breast cancer surgery: a century after Halsted. J Cancer Res Clin Oncol, 1996 122(2), 74-77. Kilkenny, JW 3rd; Bland, KI. Surgery of breast cancer. Curr Opin Oncol, 1997 9(6), 520-526. Wood, WC. Progress from clinical trials on breast cancer. Cancer, 1994 74(9 Suppl), 2606-2609. Loukas, M; Tubbs, RS; Mirzayan, N; Shirak, M; Steinberg, A; Shoja, MM. The history of mastectomy. Am Surg, 2011 77(5), 566-571. References [65] [66] [67] [68] [69] [70] [71] [72] [73] [74] [75] [76] [77] [78] [79] [80] 213 Bremers, AJ; Rutgers, EJ; van de Velde, CJ. Cancer surgery: the last 25 years. Cancer Treat Rev, 1999 25(6), 333-353. Tobias, JS. The role of radiotherapy in the management of cancer – An overview. Ann Acad Med Singapore, 1996 25(3), 371-379. Akram, M; Siddiqui, SA. Breast cancer management: past, present and evolving. Indian J Cancer, 2012 49(3), 277-282. Welbourn, RB. Highlights from endocrine surgical history. World J Surg, 1996 20(5), 603-612. Goldhirsch, A; Gelber, RD. Endocrine therapies of breast cancer. Semin Oncol, 1996 23(4), 494-505. Bilimoria, MM; Jordan, VC. Is it time to develop an optimal endocrine therapy for premenopausal patients with axillary node positive and negative breast cancer? Semin Surg Oncol, 1996 12(5), 339-345. Sheth, SP; Allegra, JC. What role for concurrent chemohormonal therapy in breast cancer? Oncology (Williston Park), 1987 1(8), 19-27. Baum, M; Demicheli, R; Hrushesky, W; Retsky, M. Does surgery unfavourably perturb the “natural history” of early breast cancer by accelerating the appearance of distant metastases? Eur J Cancer, 2005 41(4), 508-515. Ekmektzoglou, KA; Xanthos, T; German, V; Zografos, GC. Breast cancer: from the earliest times through to the end of the 20th century. Eur J Obstet Gynecol Reprod Biol, 2009 145(1), 3-8. Baum, M. Breast cancer 2000 BC to 2000 AD – Time for a paradigm shift? Acta Oncol, 1993 32(1), 3-8. von Smitten, K. Surgical management of breast cancer in the future. Acta Oncol, 2000 39(3), 437-439. Nassar, A. Core needle biopsy versus fine needle aspiration biopsy in breast – A historical perspective and opportunities in the modern era. Diagn Cytopathol, 2011 39(5), 380-388. Karacalar, A. The Amazons and an analysis of breast mutilation from a plastic surgeon’s perspective. Plast Reconstr Surg, 2007 119(3), 810-818. Rozen, WM; Rajkomar, AK; Anavekar, NS; Ashton, MW. Post-mastectomy breast reconstruction: a history in evolution. Clin Breast Cancer, 2009 9(3), 145154. Teimourian, B; Adham, MN. Louis Ombredanne and the origin of muscle flap use for immediate breast mound reconstruction. Plast Reconstr Surg, 1983 72(6), 905-910. Champaneria, MC; Wong, WW; Hill, ME; Gupta, SC. The evolution of breast reconstruction: a historical perspective. World J Surg, 2012 36(4), 730-742. 214 [81] [82] [83] [84] [85] [86] [87] [88] [89] [90] [91] [92] [93] [94] [95] [96] [97] References Beekman, WH; Hage, JJ; Jorna, LB; Mulder, JW. Augmentation mammaplasty: the story before the silicone bag prosthesis. Ann Plast Surg, 1999 43(4), 446451. Calobrace, MB; Capizzi, PJ. The biology and evolution of cohesive gel and shaped implants. Plast Reconstr Surg, 2014 134(1 Suppl), 6S-11S. Hinderer, UT; del Rio, JL. Erich Lexer’s mammaplasty. Aesthetic Plast Surg, 1992 16(2), 101-107. Evans, WP. Breast masses. Appropriate evaluation. Radiol Clin North Am, 1995 33(6), 1085-1108. Hindle, WH. The diagnostic evaluation. Obstet Gynecol Clin North Am, 1994 21(3), 499-517. Isaacs, JH. Breast biopsy and the surgical treatment of early carcinoma of the breast. Obstet Gynecol Clin North Am, 1987 14(3), 711-732. Leis, HP Jr. Current methods for biopsy and treatment of potentially curable breast cancer. Int Surg, 1990 75(1), 1-7. Chalas, E; Valea, F. The gynecologist and surgical procedures for breast disease. Clin Obstet Gynecol, 1994 37(4), 948-953. Schepps, B; Scola, FH; Frates, RE. Benign circumscribed breast masses. Mammographic and sonographic appearance. Obstet Gynecol Clin North Am, 1994 21(3), 519-537. Robidoux, A. Recent trends in the management of breast cancer. 2. Occult breast lesions: when and how to perform a biopsy for mammographic abnormalities. Can J Surg, 1992 35(4), 366-370. Gallager, HS. Breast specimen radiography. Obligatory, adjuvant and investigative. Am J Clin Pathol, 1975 64(6), 749-755. Winchester, DP. Evaluation and management of breast abnormalities. Cancer, 1990 66(6 Suppl), 1345-1347. Monsees, BS. Evaluation of breast microcalcifications. Radiol Clin North Am, 1995 33(6), 1109-1121. Donegan, WL. Evaluation of a palpable breast mass. N Engl J Med, 1992 327(13), 937-942. Schwartz, GF; Feig, SA. Management of clinically occult (nonpalpable) breast lesions. Obstet Gynecol Clin North Am, 1994 21(4), 621-637. Kurtzman, SH; MacGillivray, DC; Deckers, PJ. Evolving strategies for the management of non-palpable breast abnormalities. Surg Oncol, 1995 4(1), 1-14. Leong, AS; Mower, GA. The role of the surgical pathologist in the examination of the non-palpable breast lesion. Pathology, 1992 24(4), 264-271. References [98] [99] [100] [101] [102] [103] [104] [105] [106] [107] [108] [109] [110] [111] [112] [113] [114] 215 Preece, PE; Hunter, SM; Duguid, HL; Wood, RA. Cytodiagnosis and other methods of biopsy in the modern management of breast cancer. Semin Surg Oncol, 1989 5(2), 69-81. Layfield, LJ; Glasgow, BJ; Cramer, H. Fine-needle aspiration in the management of breast masses. Pathol Annu, 1989 24 Pt 2, 23-62. Wilkinson, EJ; Hendricks, JB. Fine needle aspiration of the breast for diagnosis of preinvasive neoplasia. J Cell Biochem Suppl, 1993 17G, 81-88. Ljung, BM; Chew, K; Deng, G; Matsumura, K; Waldman, F; Smith, H. Fine needle aspiration techniques for the characterization of breast cancers. Cancer, 1994 74(3 Suppl), 1000-1005. Millis, RR. Needle biopsy of the breast. Monogr Pathol, 1984 (25), 186-203. Zagorianakou, P; Fiaccavento, S; Zagorianakou, N; Makrydimas, G; Stefanou, D; Agnantis, NJ. FNAC: its role, limitations and perspective in the preoperative diagnosis of breast cancer. Eur J Gynaecol Oncol, 2005 26(2), 143-149. Gerhard, R; Schmitt, FC. Liquid-based cytology in fine-needle aspiration of breast lesions: a review. Acta Cytol, 2014 58(6), 533-542. Bilous, M. Breast core needle biopsy: issues and controversies. Mod Pathol, 2010 23 Suppl 2, S36-S45. Usami, S; Moriya, T; Kasajima, A; Suzuki, A; Ishida, T; Sasano, H; Ohuchi, N. Pathological aspects of core needle biopsy for non-palpable breast lesions. Breast Cancer, 2005 12(4), 272-278. Thomassin-Naggara, I; Lalonde, L; David, J; Darai, E; Uzan, S; Trop, I. A plea for the biopsy marker: how, why and why not clipping after breast biopsy? Breast Cancer Res Treat, 2012 132(3), 881-893. Lucas, JH; Cone, DL. Breast cyst aspiration. Am Fam Physician, 2003 68(10), 1983-1986. Dooley, WC. Ductal lavage, nipple aspiration, and ductoscopy for breast cancer diagnosis. Curr Oncol Rep, 2003 5(1), 63-65. Khan, SA. The role of ductal lavage in the management of women at high risk for breast carcinoma. Curr Treat Options Oncol, 2004 5(2), 145-151. Newman, LA; Blake, C. Ductal lavage for breast cancer risk assessment. Cancer Control, 2002 9(6), 473-479. Kenney, PJ; Ellison, MC. Ductal lavage in the screening of high-risk women. Curr Womens Health Rep, 2003 3(2), 151-155. Locke, I; Mitchell, G; Eeles, R. Ductal approaches to assessment and management of women at high risk for developing breast cancer. Breast Cancer Res, 2004 6(2), 75-81. Rivers, A; Newman, LA. Ductal lavage for breast cancer risk assessment. Surg Oncol Clin N Am, 2005 14(1), 45-68. 216 [115] [116] [117] [118] [119] [120] [121] [122] [123] [124] [125] [126] [127] References King, BL; Love, SM. The intraductal approach to the breast: raison d’être. Breast Cancer Res, 2006 8(2), 206. Dua, RS; Isacke, CM; Gui, GP. The intraductal approach to breast cancer biomarker discovery. J Clin Oncol, 2006 24(7), 1209-1216. Massihnia, D; Perez, A; Bazan, V; Bronte, G; Castiglia, M; Fanale, D; Barraco, N; Cangemi, A; Di Piazza, F; Calò, V; Rizzo, S; Cicero, G; Pantuso, G; Russo, A. A headlight on liquid biopsies: a challenging tool for breast cancer management. Tumour Biol, 2016 37(4), 4263-4273. De Mattos-Arruda, L; Caldas, C. Cell-free circulating tumour DNA as a liquid biopsy in breast cancer. Mol Oncol, 2016 10(3), 464-474. Guinebretière, JM; Becette, V; Hagay, C; Belichard, C; Tardivon, A; Vanel, D. Use of radiology for the pathologist in the management of breast lesions. Eur J Radiol, 2005 54(1), 15-25. Parker, SH; Stavros, AT; Dennis, MA. Needle biopsy techniques. Radiol Clin North Am, 1995 33(6), 1171-1186. The palpable breast lump: information and recommendations to assist decisionmaking when a breast lump is detected. The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Canadian Association of Radiation Oncologists. CMAJ, 1998 158 Suppl 3, S3-S8. Verkooijen, HM; Peeters, PH; Buskens, E; Koot, VC; Borel Rinkes, IH; Mali, WP; van Vroonhoven, TJ. Diagnostic accuracy of large-core needle biopsy for nonpalpable breast disease: a meta-analysis. Br J Cancer, 2000 82(5), 10171021. Kocjan, G; Bourgain, C; Fassina, A; Hagmar, B; Herbert, A; Kapila, K; KardumSkelin, I; Kloboves-Prevodnik, V; Krishnamurthy, S; Koutselini, H; Majak, B; Olszewski, W; Onal, B; Pohar-Marinsek, Z; Shabalova, I; Smith, J; Tani, E; Vielh, P; Wiener, H; Schenck, U; Schmitt, F. The role of breast FNAC in diagnosis and clinical management: a survey of current practice. Cytopathology, 2008 19(5), 271-278. Shao, ZM; Nguyen, M. Nipple aspiration in diagnosis of breast cancer. Semin Surg Oncol, 2001 20(3), 175-180. Albanghali, M; Green, A; Rakha, E; Aleskandarany, M; Nolan, C; Ellis, I; Cheung, KL. Construction of tissue microarrays from core needle biopsies – A systematic literature review. Histopathology, 2016 68(3), 323-332. Calhoun, BC; Collins, LC. Recommendations for excision following core needle biopsy of the breast: a contemporary evaluation of the literature. Histopathology, 2016 68(1), 138-151. Masood, S. Development of a novel approach for breast cancer prediction and early detection using minimally invasive procedures and molecular analysis: how References [128] [129] [130] [131] [132] [133] [134] [135] [136] [137] [138] [139] [140] [141] [142] 217 cytomorphology became a breast cancer risk predictor. Breast J, 2015 21(1), 8296. Neal, L; Sandhu, NP; Hieken, TJ; Glazebrook, KN; Mac Bride, MB; Dilaveri, CA; Wahner-Roedler, DL; Ghosh, K; Visscher, DW. Diagnosis and management of benign, atypical, and indeterminate breast lesions detected on core needle biopsy. Mayo Clin Proc, 2014 89(4), 536-547. Simsir, A; Cangiarella, J. Challenging breast lesions: pitfalls and limitations of fine-needle aspiration and the role of core biopsy in specific lesions. Diagn Cytopathol, 2012 40(3), 262-272. Levin, DC; Parker, L; Schwartz, GF; Rao, VM. Percutaneous needle vs surgical breast biopsy: previous allegations of overuse of surgery are in error. J Am Coll Radiol, 2012 9(2), 137-140. Landercasper, J; Linebarger, JH. Contemporary breast imaging and concordance assessment: a surgical perspective. Surg Clin North Am, 2011 91(1), 33-58. Kooistra, B; Wauters, C; Strobbe, L; Wobbes, T. Preoperative cytological and histological diagnosis of breast lesions: A critical review. Eur J Surg Oncol, 2010 36(10), 934-940. Georgian-Smith, D; Lawton, TJ. Controversies on the management of high-risk lesions at core biopsy from a radiology/pathology perspective. Radiol Clin North Am, 2010 48(5), 999-1012. Litherland, JC. Should fine needle aspiration cytology in breast assessment be abandoned? Clin Radiol, 2002 57(2), 81-84. Sneige, N. Utility of cytologic specimens in the evaluation of prognostic and predictive factors of breast cancer: current issues and future directions. Diagn Cytopathol, 2004 30(3), 158-165. Suijkerbuijk, KP; van der Wall, E; Vooijs, M; van Diest, PJ. Molecular analysis of nipple fluid for breast cancer screening. Pathobiology, 2008 75(2), 149-152. Osuch, JR. Benign lesions of the breast other than fibrocystic change. Obstet Gynecol Clin North Am, 1987 14(3), 703-710. Marchant, DJ. Benign breast disease. Obstet Gynecol Clin North Am, 2002 29(1), 1-20. Marchant, DJ. Controversies in benign breast disease. Surg Oncol Clin N Am, 1998 78(2), 285-298. Morrow, M. The evaluation of common breast problems. Am Fam Physician, 2000 61(8), 2371-2378, 2385. Norris, TG. Benign breast disease. Radiol Technol, 2001 72(3), 245-267. Hamed, H; Fentiman, IS. Benign breast disease. Int J Clin Pract, 2001 55(7), 461-464. 218 [143] [144] [145] [146] [147] [148] [149] [150] [151] [152] [153] [154] [155] [156] [157] References Hughes, LE. Benign breast disorders: the clinician’s view. Cancer Detect Prev, 1992 16(1), 1-5. Sickles, EA. Management of probably benign breast lesions. Radiol Clin North Am, 1995 33(6), 1123-1130. Neal, L; Tortorelli, CL; Nassar, A. Clinician’s guide to imaging and pathologic findings in benign breast disease. Mayo Clin Proc, 2010 85(3), 274-279. Forrest, AP. Benign breast disease: end-points of treatment. Cancer Detect Prev, 1992 16(1), 61-65. Lavoué, V; Fritel, X; Antoine, M; Beltjens, F; Bendifallah, S; Boisserie-Lacroix, M; Boulanger, L; Canlorbe, G; Catteau-Jonard, S; Chabbert-Buffet, N; Chamming’s, F; Chéreau, E; Chopier, J; Coutant, C; Demetz, J; Guilhen, N; Fauvet, R; Kerdraon, O; Laas, E; Legendre, G; Mathelin, C; Nadeau, C; Naggara, IT; Ngô, C; Ouldamer, L; Rafii, A; Roedlich, MN; Seror, J; Séror, JY; Touboul, C; Uzan, C; Daraï, E; French College of Gynecologists and Obstetricians (CNGOF). Clinical practice guidelines from the French College of Gynecologists and Obstetricians (CNGOF): benign breast tumors – Short text. Eur J Obstet Gynecol Reprod Biol, 2016 200, 16-23. Pryor, LS; Lehman, JA Jr; Workman, MC. Disorders of the female breast in the pediatric age group. Plast Reconstr Surg, 2009 124(1 Suppl), 50e-60e. van Aalst, JA; Sadove, AM. Treatment of pediatric breast problems. Clin Plast Surg, 2005 32(1), 65-78. van Aalst, JA; Phillips, JD; Sadove, AM. Pediatric chest wall and breast deformities. Plast Reconstr Surg, 2009 124(1 Suppl), 38e-49e. Neinstein, LS. Breast disease in adolescents and young women. Pediatr Clin North Am, 1999 46(3), 607-629. Simmons, PS. Diagnostic considerations in breast disorders of children and adolescents. Obstet Gynecol Clin North Am, 1992 19(1), 91-102. Chang, DS; McGrath, MH. Management of benign tumors of the adolescent breast. Plast Reconstr Surg, 2007 120(1), 13e-19e. ACOG Committee on Adolescent Health Care. ACOG Committee Opinion No. 350, November 2006: Breast concerns in the adolescent. Obstet Gynecol, 2006 108(5), 1329-1336. Lemaine, V; Simmons, PS. The adolescent female: Breast and reproductive embryology and anatomy. Clin Anat, 2013 26(1), 22-28. Rahman, GA; Adigun, IA; Yusuf, IF. Macromastia: a review of presentation and management. Niger Postgrad Med J, 2010 17(1), 45-49. Dancey A, Khan M, Dawson J, Peart F. Gigantomastia – A classification and review of the literature. J Plast Reconstr Aesthet Surg, 2008 61(5), 493-502. References [158] [159] [160] [161] [162] [163] [164] [165] [166] [167] [168] [169] [170] [171] [172] [173] [174] [175] [176] 219 Corriveau, S; Jacobs, JS. Macromastia in adolescence. Clin Plast Surg, 1990 17(1), 151-160. Hoppe, IC; Patel, PP; Singer-Granick, CJ; Granick, MS. Virginal mammary hypertrophy: a meta-analysis and treatment algorithm. Plast Reconstr Surg, 2011 127(6), 2224-2231. Gumm, R; Cunnick, GH; Mokbel, K. Evidence for the management of mastalgia. Curr Med Res Opin, 2004 20(5), 681-684. Smith, RL; Pruthi, S; Fitzpatrick, LA. Evaluation and management of breast pain. Mayo Clin Proc, 2004 79(3), 353-372. Millet, AV; Dirbas, FM. Clinical management of breast pain: a review. Obstet Gynecol Surv, 2002 57(7), 451-461 BeLieu, RM. Mastodynia. Obstet Gynecol Clin North Am, 1994 21(3), 461-477. Maddox, PR; Mansel, RE. Management of breast pain and nodularity. World J Surg, 1989 13(6), 699-705. Latham, K; Fernandez, S; Iteld, L; Panthaki, Z; Armstrong, MB; Thaller, S. Pediatric breast deformity. J Craniofac Surg, 2006 17(3), 454-467. Kulkarni, D; Dixon, JM. Congenital abnormalities of the breast. Womens Health (Lond), 2012 8(1), 75-86. Urschel, HC Jr. Poland syndrome. Semin Thorac Cardiovasc Surg, 2009 21(1), 89-94. Moir, CR; Johnson, CH. Poland’s syndrome. Semin Pediatr Surg, 2008 17(3), 161-166. Loukas, M; Clarke, P; Tubbs, RS. Accessory breasts: a historical and current perspective. Am Surg, 2007 73(5), 525-528. Newman, M. Supernumerary nipples. Am Fam Physician, 1988 38(2), 183-188. Sillesen, NH; Hölmich, LR; Siersen, HE; Bonde, C. Congenital symmastia revisited. J Plast Reconstr Aesthet Surg, 2012 65(12), 1607-1613. Ritz, M; Silfen, R; Morgan, D; Southwick, G. Simple technique for inverted nipple correction. Aesthetic Plast Surg, 2005 29(1), 24-27. Costagliola, M; Atiyeh, B; Rampillon, F. Tuberous breast: revised classification and a new hypothesis for its development. Aesthetic Plast Surg, 2013 37(5), 896903. Hughes, LE. Non-lactational inflammation and duct ectasia. Br Med Bull, 1991 47(2), 272-283. Stone, K; Wheeler, A. A Review of Anatomy, Physiology, and Benign Pathology of the Nipple. Ann Surg Oncol, 2015 22(10), 3236-3240. Spyropoulou, GA; Pavlidis, L; Trakatelli, M; Athanasiou, E; Pazarli, E; Sotiriadis, D; Demiri, E. Rare benign tumours of the nipple. J Eur Acad Dermatol Venereol, 2015 29(1), 7-13. 220 [177] [178] [179] [180] [181] [182] [183] [184] [185] [186] [187] [188] [189] [190] [191] [192] [193] [194] References Vianna, LL; Millis, RR; Fentiman, IS. Adenoma of the nipple: a diagnostic dilemma. Br J Hosp Med, 1993-1994 50(11), 639-642. Oo, KZ; Xiao, PQ. Infiltrating syringomatous adenoma of the nipple: clinical presentation and literature review. Arch Pathol Lab Med, 2009 133(9), 14871489. Lang, JE; Kuerer, HM. Breast ductal secretions: clinical features, potential uses, and possible applications. Cancer Control, 2007 14(4), 350-359. Okazaki, A; Hirata, K; Okazaki, M; Svane, G; Azavedo, E. Nipple discharge disorders: current diagnostic management and the role of fiber-ductoscopy. Eur Radiol, 1999 9(4), 583-590. Vargas, HI; Romero, L; Chlebowski, RT. Management of bloody nipple discharge. Curr Treat Options Oncol, 2002 3(2), 157-161. Sakorafas, GH. Nipple discharge: current diagnostic and therapeutic approaches. Cancer Treat Rev, 2001 27(5), 275-282. Ingvar, C. Papillary secretion. Diagnostic assessment and treatment. Scand J Surg, 2002 91(3), 246-250. Irusen, H; Rohwer, AC; Steyn, DW; Young, T. Treatments for breast abscesses in breastfeeding women. Cochrane Database Syst Rev, 2015 (8), CD010490. Trop, I; Dugas, A; David, J; El Khoury, M; Boileau, JF; Larouche, N; Lalonde, L. Breast abscesses: evidence-based algorithms for diagnosis, management, and follow-up. Radiographics, 2011 31(6), 1683-1699. Dixon, JM. Periductal mastitis/duct ectasia. World J Surg, 1989 13(6), 715-720. Diesing, D; Axt-Fliedner, R; Hornung, D; Weiss, JM; Diedrich, K; Friedrich, M. Granulomatous mastitis. Arch Gynecol Obstet, 2004 269(4), 233-236. Benson, JR; Dumitru, D. Idiopathic granulomatous mastitis: presentation, investigation and management. Future Oncol, 2016 12(11), 1381-1394. Gon, S; Bhattacharyya, A; Majumdar, B; Kundu, S. Tubercular mastitis – A great masquerader. Turk Patoloji Derg, 2013 29(1), 61-63. Dixon, JM. Cystic disease and fibroadenoma of the breast: natural history and relation to breast cancer risk. Br Med Bull, 1991 47(2), 258-271. Jayasinghe, Y; Simmons, PS. Fibroadenomas in adolescence. Curr Opin Obstet Gynecol, 2009 21(5), 402-406. Musio, F; Mozingo, D; Otchy, DP. Multiple, giant fibroadenoma. Am Surg, 1991 57(7), 438-441. Greenberg, R; Skornick, Y; Kaplan, O. Management of breast fibroadenomas. J Gen Intern Med, 1998 13(9), 640-645. Matherne, TH; Green, A Jr; Tucker, JA; Dyess, DL. Fibromatosis: the breast cancer imitator. South Med J, 2004 97(11), 1100-1103. References [195] [196] [197] [198] [199] [200] [201] [202] [203] [204] [205] [206] [207] [208] [209] [210] [211] 221 Ebrahim, L; Parry, J; Taylor, DB. Fibromatosis of the breast: a pictorial review of the imaging and histopathology findings. Clin Radiol, 2014 69(10), 10771083. Fiorica, JV. Fibrocystic changes. Obstet Gynecol Clin North Am, 1994 21(3), 445-452. Drukker, BH; deMendonca, WC. Fibrocystic change and fibrocystic disease of the breast. Obstet Gynecol Clin North Am, 1987 14(3), 685-702. Wells, CA; El-Ayat, GA. Non-operative breast pathology: apocrine lesions. J Clin Pathol, 2007 60(12), 1313-1320. Haagensen, DE Jr. Is cystic disease related to breast cancer? Am J Surg Pathol, 1991 15(7), 687-694. Heisey, R; Mahoney, L; Watson, B. Management of palpable breast lumps. Consensus guideline for family physicians. Can Fam Physician, 1999 45, 19261932. Jagmohan, P; Pool, FJ; Putti, TC; Wong, J. Papillary lesions of the breast: imaging findings and diagnostic challenges. Diagn Interv Radiol, 2013 19(6), 471-478. Ibarra, JA. Papillary lesions of the breast. Breast J, 2006 12(3), 237-251. Batchelor, JS; Farah, G; Fisher, C. Multiple breast papillomas in adolescence. J Surg Oncol, 1993 54(1), 64-66. Collins, LC; Schnitt, SJ. Papillary lesions of the breast: selected diagnostic and management issues. Histopathology, 2008 52(1), 20-29. Agoumi, M; Giambattista, J; Hayes, MM. Practical Considerations in Breast Papillary Lesions: A Review of the Literature. Arch Pathol Lab Med, 2016 140(8), 770-790. Ueng, SH; Mezzetti, T; Tavassoli, FA. Papillary neoplasms of the breast: a review. Arch Pathol Lab Med, 2009 133(6), 893-907. Murray, M. Nonneoplastic alterations of the mammary epithelium can mimic atypia. Arch Pathol Lab Med, 2009 133(5), 722-728. Kiluk, JV; Acs, G; Hoover, SJ. High-risk benign breast lesions: current strategies in management. Cancer Control, 2007 14(4), 321-329. Masood, S. Why the term “low-grade ductal carcinoma in situ” should be changed to “borderline breast disease”: diagnostic and clinical implications. Womens Health (Lond), 2012 8(1), 57-62. Simmons, RM; Osborne, MP. The evaluation of high risk and pre-invasive breast lesions and the decision process for follow up and surgical intervention. Surg Oncol, 1999 8(2), 55-65. Masood, S; Rosa, M. Borderline breast lesions: diagnostic challenges and clinical implications. Adv Anat Pathol, 2011 18(3), 190-198. 222 [212] [213] [214] [215] [216] [217] [218] [219] [220] [221] [222] [223] [224] [225] [226] [227] References Tavassoli, FA. Ductal carcinoma in situ: introduction of the concept of ductal intraepithelial neoplasia. Mod Pathol, 1998 11(2), 140-154. Dion, L; Racin, A; Brousse, S; Beltjens, F; Cauchois, A; Levêque, J; Coutant, C; Lavoué, V. Atypical epithelial hyperplasia of the breast: state of the art. Expert Rev Anticancer Ther, 2016 16(9), 943-953. Nasser, SM. Flat epithelial atypia of the breast. J Med Liban, 2009 57(2), 105109. Lerwill, MF. Flat epithelial atypia of the breast. Arch Pathol Lab Med, 2008 132(4), 615-621. Sudarshan, M; Meguerditchian, AN; Mesurolle, B; Meterissian, S. Flat epithelial atypia of the breast: characteristics and behaviors. Am J Surg, 2011 201(2), 245250. Jara-Lazaro, AR; Tse, GM; Tan, PH. Columnar cell lesions of the breast: an update and significance on core biopsy. Pathology, 2009 41(1), 18-27. Clauser, P; Marino, MA; Baltzer, PA; Bazzocchi, M; Zuiani, C. Management of atypical lobular hyperplasia, atypical ductal hyperplasia, and lobular carcinoma in situ. Expert Rev Anticancer Ther, 2016 16(3), 335-346. Pinder, SE; Reis-Filho, JS. Non-operative breast pathology: columnar cell lesions. J Clin Pathol, 2007 60(12), 1307-1312. Jorns, J; Sabel, MS; Pang, JC. Lobular neoplasia: morphology and management. Arch Pathol Lab Med, 2014 138(10), 1344-1349. Anderson, BO; Calhoun, KE; Rosen, EL. Evolving concepts in the management of lobular neoplasia. J Natl Compr Canc Netw, 2006 4(5), 511-522. Reis-Filho, JS; Pinder, SE. Non-operative breast pathology: lobular neoplasia. J Clin Pathol, 2007 60(12), 1321-1327. King, TA; Reis-Filho, JS. Lobular neoplasia. Surg Oncol Clin N Am, 2014 23(3), 487-503. Purushothaman, HN; Lekanidi, K; Shousha, S; Wilson, R. Lesions of uncertain malignant potential in the breast (B3): what do we know? Clin Radiol, 2016 71(2), 134-140. Morrow, M; Schnitt, SJ; Norton, L. Current management of lesions associated with an increased risk of breast cancer. Nat Rev Clin Oncol, 2015 12(4), 227238. Lanyi, M. Microcalcifications in the breast – A blessing or a curse? A critical review. Diagn Imaging Clin Med, 1985 54(3-4), 126-145. Tavassoli, FA. Lobular and ductal intraepithelial neoplasia. Pathologe, 2008 29 Suppl 2, 107-111. References [228] [229] [230] [231] [232] [233] [234] [235] [236] [237] [238] [239] [240] [241] [242] [243] [244] 223 Pinder, SE; Provenzano, E; Reis-Filho, JS. Lobular in situ neoplasia and columnar cell lesions: diagnosis in breast core biopsies and implications for management. Pathology, 2007 39(2), 208-216. Zagouri, F; Sergentanis, TN; Zografos, GC. Precursors and preinvasive lesions of the breast: the role of molecular prognostic markers in the diagnostic and therapeutic dilemma. World J Surg Oncol, 2007 5, 57. Kennedy, M; Masterson, AV; Kerin, M; Flanagan, F. Pathology and clinical relevance of radial scars: a review. J Clin Pathol, 2003 56(10), 721-724. Costa, A; Zanini, V. Precancerous lesions of the breast. Nat Clin Pract Oncol, 2008 5(12), 700-704. Page, DL. Breast lesions, pathology and cancer risk. Breast J, 2004 10 Suppl 1, S3-S4. Ho, BC; Tan, PH. Lobular neoplasia of the breast: 68 years on. Pathology, 2009 41(1), 28-35. Simpson, JF. Update on atypical epithelial hyperplasia and ductal carcinoma in situ. Pathology, 2009 41(1), 36-39. Degnim, AC; King, TA. Surgical management of high-risk breast lesions. Surg Clin North Am, 2013 93(2), 329-340. Virk, RK; Khan, A. Pseudoangiomatous stromal hyperplasia: an overview. Arch Pathol Lab Med, 2010 134(7), 1070-1074. AbdullGaffar, B. Pseudoangiomatous stromal hyperplasia of the breast. Arch Pathol Lab Med, 2009 133(8), 1335-1338. Khan, SA; Badve, S. Phyllodes tumors of the breast. Curr Treat Options Oncol, 2001 2(2), 139-147. Parker, SJ; Harries, SA. Phyllodes tumours. Postgrad Med J, 2001 77(909), 428435. Reinfuss, M; Mitus, J; Stelmach, A. Phyllodes tumor of the breast. Strahlenther Onkol, 1995 171(1), 5-11. Plaza, MJ; Swintelski, C; Yaziji, H; Torres-Salichs, M; Esserman, LE. Phyllodes tumor: review of key imaging characteristics. Breast Dis, 2015 35(2), 79-86. Liang, MI; Ramaswamy, B; Patterson, CC; McKelvey, MT; Gordillo, G; Nuovo, GJ; Carson, WE 3rd. Giant breast tumors: surgical management of phyllodes tumors, potential for reconstructive surgery and a review of literature. World J Surg Oncol, 2008 6, 117. Jacklin, RK; Ridgway, PF; Ziprin, P; Healy, V; Hadjiminas, D; Darzi, A. Optimising preoperative diagnosis in phyllodes tumour of the breast. J Clin Pathol, 2006 59(5), 454-459. Magro, G. Mammary myofibroblastoma: a tumor with a wide morphologic spectrum. Arch Pathol Lab Med, 2008 132(11), 1813-1820. 224 [245] [246] [247] [248] [249] [250] [251] [252] [253] [254] [255] [256] [257] [258] [259] [260] References Tan, PH; Lai, LM; Carrington, EV; Opaluwa, AS; Ravikumar, KH; Chetty, N; Kaplan, V; Kelley, CJ; Babu, ED. Fat necrosis of the breast – A review. Breast, 2006 15(3), 313-318. Riley, MP; Karamchandani, DM. Mammary Hibernoma: A Rare Entity. Arch Pathol Lab Med, 2015 139(12), 1565-1567. Thorncroft, K; Forsyth, L; Desmond, S; Audisio, RA. The diagnosis and management of diabetic mastopathy. Breast J, 2007 13(6), 607-613. Kudva, YC; Reynolds, CA; O’Brien, T; Crotty, TB. Mastopathy and diabetes. Curr Diab Rep, 2003 3(1), 56-59. Haj, M; Weiss, M; Herskovits, T. Diabetic sclerosing lymphocytic lobulitis of the breast. J Diabetes Complications, 2004 18(3), 187-191. Monypenny, IJ. Surgical assessment of benign breast disease in screening. Br J Clin Pract Suppl, 1989 68, 118-20. Xing, L; He, Q; Wang, YY; Li, HY; Ren, GS. Advances in the surgical treatment of breast cancer. Chin Clin Oncol, 2016 5(3), 34. Shahbazi, S; Woods, SJ. Influence of physician, patient, and health care system characteristics on the use of outpatient mastectomy. Am J Surg, 2016 211(4), 802-809. Euhus, DM. New Insights into the Surgical Management of Breast Cancer. Semin Radiat Oncol, 2016 26(1), 25-36. Esserman, L; Gallant, E; Alvarado, M. Less Is More: The Evolving Surgical Approach to Breast Cancer. Am Soc Clin Oncol Educ Book, 2016 35, e5-e10. Peart, O. Breast intervention and breast cancer treatment options. Radiol Technol, 2015 86(5), 535M-558M. Zurrida, S; Veronesi, U. Milestones in breast cancer treatment. Breast J, 2015 21(1), 3-12. Franceschini, G; Martin Sanchez, A; Di Leone, A; Magno, S; Moschella, F; Accetta, C; Masetti, R. New trends in breast cancer surgery: a therapeutic approach increasingly efficacy and respectful of the patient. G Chir, 2015 36(4), 145-152. Alkatout, I; Order, B; Klapper, W; Weigel, MT; Jonat, W; Schaefer, FK; Mundhenke, C; Wenners, A. Surgical impact of new treatments in breast cancer. Minerva Ginecol, 2013 65(4), 363-383. Critchley, AC; Thompson, AM; Chan, HY; Reed, MW. Current controversies in breast cancer surgery. Clin Oncol (R Coll Radiol), 2013 25(2), 101-108. Pilewskie, M; King, TA. Age and molecular subtypes: impact on surgical decisions. J Surg Oncol, 2014 110(1), 8-14. References [261] [262] [263] [264] [265] [266] [267] [268] [269] [270] [271] [272] [273] [274] [275] [276] 225 Sharma, S; Barry, M; Gallagher, DJ; Kell, M; Sacchini, V. An overview of triple negative breast cancer for surgical oncologists. Surg Oncol, 2015 24(3), 276283. Hermann, RE; Steiger, E. Modified radical mastectomy. Surg Clin North Am, 1978 58(4), 743-754. Osborne, MP; Borgen, PI. Role of mastectomy in breast cancer. Surg Clin North Am, 1990 70(5), 1023-1046. Donegan, WL. Cancer of the breast. Staging methods, primary treatment options and end results. Major Probl Clin Surg, 1979 5, 221-301. Hayes, MK. Update on Preoperative Breast Localization. Radiol Clin North Am, 2017 55(3), 591-603. Baker, RR. Preoperative assessment of the patient with breast cancer. Surg Clin North Am, 1978 58(4), 681-691. Baker, RR. Preoperative assessment of the patient with breast cancer. Surg Clin North Am, 1984 64(6), 1039-1050. Recht, A; Connolly, JL; Schnitt, SJ; Harris, JR. Therapy of in situ cancer. Hematol Oncol Clin North Am, 1989 3(4), 691-708. Fentiman, IS. 8. The dilemma of in situ carcinoma of the breast. Int J Clin Pract, 2001 55(10), 680-683. Shah, C; Wobb, J; Manyam, B; Kundu, N; Arthur, D; Wazer, D; Fernandez, E; Vicini, F. Management of Ductal Carcinoma In Situ of the Breast: A Review. JAMA Oncol, 2016 2(8), 1083-1088. Ahmed, M; Rubio, IT; Klaase, JM; Douek, M. Surgical treatment of nonpalpable primary invasive and in situ breast cancer. Nat Rev Clin Oncol, 2015 12(11), 645-663. Henry-Tillman, RS; Klimberg, VS. In situ breast cancer. Curr Treat Options Oncol, 2000 1(3), 199-209. Frykberg, ER; Masood, S; Copeland, EM 3rd; Bland, KI. Ductal carcinoma in situ of the breast. Surg Gynecol Obstet, 1993 177(4), 425-440. Moore, MM. treatment of ductal carcinoma in situ of the breast. Semin Surg Oncol, 1991 7(5), 267-270. Johns, N; Dixon, JM. Should patients with early breast cancer still be offered the choice of breast conserving surgery or mastectomy? Eur J Surg Oncol, 2016 42(11), 1636-1641. Mac Bride, MB; Neal, L; Dilaveri, CA; Sandhu, NP; Hieken, TJ; Ghosh, K; Wahner-Roedler, DL. Factors associated with surgical decision making in women with early-stage breast cancer: a literature review. J Womens Health (Larchmt), 2013 22(3), 236-242. 226 [277] [278] [279] [280] [281] [282] [283] [284] [285] [286] [287] [288] [289] [290] [291] [292] [293] References Lebeau, A; Kühn, T. Updates in the treatment of ductal carcinoma in situ of the breast. Curr Opin Obstet Gynecol, 2016 28(1), 49-58. Benson, JR; Jatoi, I; Keisch, M; Esteva, FJ; Makris, A; Jordan, VC. Early breast cancer. Lancet, 2009 373(9673), 1463-1479. Frykberg, ER; Santiago, F; Betsill, WL Jr; O’Brien, PH. Lobular carcinoma in situ of the breast. Surg Gynecol Obstet, 1987 164(3), 285-301. Fisher, ER; Fisher, B. Lobular carcinoma of the breast: an overview. Ann Surg, 1977 185(4), 377-385. Grooff, PN; Pamies, RJ; Hunyadi, S. Lobular carcinoma in situ: what clinicians need to know. Hosp Pract (Off Ed), 1993 28(6), 122, 125, 129-30. Goldschmidt, RA; Victor, TA. Lobular carcinoma in situ of the breast. Semin Surg Oncol, 1996 12(5), 314-320. Cocquyt, V; Van Belle, S. Lobular carcinoma in situ and invasive lobular cancer of the breast. Curr Opin Obstet Gynecol, 2005 17(1), 55-60. Afonso, N; Bouwman, D. Lobular carcinoma in situ. Eur J Cancer Prev, 2008 17(4), 312-316. Masannat, YA; Bains, SK; Pinder, SE; Purushotham, AD. Challenges in the management of pleomorphic lobular carcinoma in situ of the breast. Breast, 2013 22(2), 194-196. Wirman, JA. The clinical significance of minimal breast cancer: a pathologist’s viewpoint. Crit Rev Oncol Hematol, 1985 3(1), 35-74. Obeidat, R; Finnell, DS; Lally, RM. Decision aids for surgical treatment of early stage breast cancer: a narrative review of the literature. Patient Educ Couns, 2011 85(3), e311-e321. Sabel, MS. Surgical considerations in early-stage breast cancer: lessons learned and future directions. Semin Radiat Oncol, 2011 21(1), 10-19. van Dongen, JA. Early breast cancer. A review. Acta Oncol, 1989 28(1), 123134. Schwade, JG; Robinson, DS; Love, N. Primary localized breast cancer. Treatment options and informed choices. Postgrad Med, 1989 86(5), 181-184, 188, 191-192. Rao, R; Wiechmann, L. Treatment of early breast cancer. Minerva Endocrinol, 2009 34(4), 311-324. Kantor, O; Winchester, DJ. Breast conserving therapy for DCIS – Does size matter? J Surg Oncol, 2014 110(1), 75-81. Julian, TB; Venditti, CA; Duggal, S. Landmark clinical trials influencing surgical management of non-invasive and invasive breast cancer. Breast J, 2015 21(1), 60-66. References [294] [295] [296] [297] [298] [299] [300] [301] [302] [303] [304] [305] [306] [307] [308] [309] 227 Groen, EJ; Elshof, LE; Visser, LL; Rutgers, EJ; Winter-Warnars, HA; Lips, EH; Wesseling, J. Finding the balance between over- and under-treatment of ductal carcinoma in situ (DCIS). Breast, 2017 31, 274-283. Brill, KL; Brenin, DR. Occult breast cancer and axillary mass. Curr Treat Options Oncol, 2001 2(2), 149-155. Sakorafas, GH; Tsiotou, AG. Occult breast cancer: a challenge from a surgical perspective. Surg Oncol, 1999 8(1), 27-33. Gump, FE. Multicentricity in early breast cancer. Semin Surg Oncol, 1992 8(3), 117-121. Jain, S; Rezo, A; Shadbolt, B; Dahlstrom, JE. Synchronous multiple ipsilateral breast cancers: implications for patient management. Pathology, 2009 41(1), 5767. Bendifallah, S; Werkoff, G; Borie-Moutafoff, C; Antoine, M; Chopier, J; Gligorov, J; Uzan, S; Coutant, C; Rouzier, R. Multiple synchronous (multifocal and multicentric) breast cancer: clinical implications. Surg Oncol, 2010 19(4), e115-e123. Patani, N; Carpenter, R. Oncological and aesthetic considerations of conservational surgery for multifocal/multicentric breast cancer. Breast J, 2010 16(3), 222-232. Tan, MP. A Novel Segment Classification for Multifocal and Multicentric Breast Cancer to Facilitate Breast-Conservation Treatment. Breast J, 2015 21(4), 410417. Milulescu, A; Di Marino, L; Peradze, N; Toesca, A. Management of MultifocalMulticentric Breast Cancer: Current Perspective. Chirurgia (Bucur), 2017 112(1), 12-17. Sarnelli, R; Squartini, F. Multicentricity in breast cancer: a submacroscopic study. Pathol Annu, 1986 21 Pt 1, 143-158. Narod, SA. Bilateral breast cancers. Nat Rev Clin Oncol, 2014 11(3), 157-166. Fracchia, AA; Borgen, PI. Bilateral breast cancer. Semin Surg Oncol, 1991 7(5), 300-305. Leis, HP Jr. Bilateral breast cancer. Surg Clin North Am, 1978 58(4), 833-841. Dawson, LA; Chow, E; Goss, PE. Evolving perspectives in contralateral breast cancer. Eur J Cancer, 1998 34(13), 2000-2009. Simos, D; Clemons, M; Ginsburg, OM; Jacobs, C. Definition and consequences of locally advanced breast cancer. Curr Opin Support Palliat Care, 2014 8(1), 33-38. Eroğlu, A; Aydin, F. Management of non-inflammatory locally advanced breast cancer: focus on surgical approaches. Exp Oncol, 2013 35(4), 272-279. 228 [310] [311] [312] [313] [314] [315] [316] [317] [318] [319] [320] [321] [322] [323] [324] [325] References Wilson, RE. Recommendations for the surgical management of advanced breast cancer. Oncology (Williston Park), 1987 1(3), 21-26. Black, DM; Mittendorf, EA. Landmark trials affecting the surgical management of invasive breast cancer. Surg Clin North Am, 2013 93(2), 501-518. Rashid, OM; Takabe, K. Does removal of the primary tumor in metastatic breast cancer improve survival? J Womens Health (Larchmt), 2014 23(2), 184-188. Lang, JE; Babiera, GV. Locoregional Resection in Stage IV breast cancer: tumor biology, molecular and clinical perspectives. Surg Clin North Am, 2007 87(2), 527-538. Criscitiello, C; Giuliano, M; Curigliano, G; De Laurentiis, M; Arpino, G; Carlomagno, N; De Placido, S; Golshan, M; Santangelo, M. Surgery of the primary tumor in de novo metastatic breast cancer: To do or not to do? Eur J Surg Oncol, 2015 41(10), 1288-1292. Patrick, J; Khan, SA. Surgical management of de novo stage IV breast cancer. J Natl Compr Canc Netw, 2015 13(4), 487-493. Khan, SA. De novo Stage IV breast cancer: breast conserving resection of the primary tumor? J Surg Oncol, 2014 110(1), 51-57. Khan, SA. Surgical Management of de novo Stage IV Breast Cancer. Semin Radiat Oncol, 2016 26(1), 79-86. Gradishar, WJ. Treatment of metastatic breast cancer. J Natl Compr Canc Netw, 2014 12(5 Suppl), 759-761. D’Aiuto, M; Cicalese, M; D’Aiuto, G; Rocco, G. Surgery of the chest wall for involvement by breast cancer. Thorac Surg Clin, 2010 20(4), 509-517. Sepesi, B. Management of Breast Cancer Invading Chest Wall. Thorac Surg Clin, 2017 27(2), 159-163. Ellis, LM; Bland, KI; Copeland, EM 3rd. Inflammatory breast cancer: advances in therapy. Semin Surg Oncol, 1988 4(4), 261-267. Dushkin, H; Cristofanilli, M. Inflammatory breast cancer. J Natl Compr Canc Netw, 2011 9(2), 233-240. Grace, WR; Cooperman, AM. Inflammatory breast cancer. Surg Clin North Am, 1985 65(1), 151-160. Yamauchi, H; Woodward, WA; Valero, V; Alvarez, RH; Lucci, A; Buchholz, TA; Iwamoto, T; Krishnamurthy, S; Yang, W; Reuben, JM; Hortobágyi, GN; Ueno, NT. Inflammatory breast cancer: what we know and what we need to learn. Oncologist, 2012 17(7), 891-899. van Uden, DJ; van Laarhoven, HW; Westenberg, AH; de Wilt, JH; BlankenPeeters, CF. Inflammatory breast cancer: an overview. Crit Rev Oncol Hematol, 2015 93(2), 116-126. References [326] [327] [328] [329] [330] [331] [332] [333] [334] [335] [336] [337] [338] [339] [340] [341] [342] 229 Saremian, J; Rosa, M. Solid papillary carcinoma of the breast: a pathologically and clinically distinct breast tumor. Arch Pathol Lab Med, 2012 136(10), 13081311. George, K; Anna, Z; Evanthia, K; Vassilios, K. Encapsulated papillary carcinoma of the breast: An overview. J Cancer Res Ther, 2013 9(4), 564-570. Lopes Filho, LL; Lopes, IM; Lopes, LR; Enokihara, MM; Michalany, AO; Matsunaga, N. Mammary and extramammary Paget’s disease. An Bras Dermatol, 2015 90(2), 225-231. Sandoval-Leon, AC; Drews-Elger, K; Gomez-Fernandez, CR; Yepes, MM; Lippman, ME. Paget’s disease of the nipple. Breast Cancer Res Treat, 2013 141(1), 1-12. Helme, S; Harvey, K; Agrawal, A. Breast-conserving surgery in patients with Paget’s disease. Br J Surg, 2015 102(10), 1167-1174. Sakorafas, GH; Blanchard, DK; Sarr, MG; Farley, DR. Paget’s disease of the breast: a clinical perspective. Langenbecks Arch Surg, 2001 386(6), 444-450. Sakorafas, GH; Blanchard, K; Sarr, MG; Farley, DR. Paget’s disease of the breast. Cancer Treat Rev, 2001 27(1), 9-18. Jamali, FR; Ricci, A Jr; Deckers, PJ. Paget’s disease of the nipple-areola complex. Surg Clin North Am, 1996 76(2), 365-381. Lahat, G; Lev, D; Gerstenhaber, F; Madewell, J; Le-Petross, H; Pollock, RE. Sarcomas of the breast. Expert Rev Anticancer Ther, 2012 12(8), 1045-1051. Moore, MP; Kinne, DW. Breast sarcoma. Surg Clin North Am, 1996 76(2), 383392. Trent II, JC 2nd; Benjamin, RS; Valero, V. Primary soft tissue sarcoma of the breast. Curr Treat Options Oncol, 2001 2(2), 169-176. Nizri, E; Merimsky, O; Lahat G. Optimal management of sarcomas of the breast: an update. Expert Rev Anticancer Ther, 2014 14(6), 705-710. Hsu, C; McCloskey, SA; Peddi, PF. Management of Breast Sarcoma. Surg Clin North Am, 2016 96(5), 1047-1058. Lim, SZ; Ong, KW; Tan, BK; Selvarajan, S; Tan, PH. Sarcoma of the breast: an update on a rare entity. J Clin Pathol, 2016 69(5), 373-381. Williams, EV; Banerjee, D; Dallimore, N; Monypenny, IJ. Angiosarcoma of the breast following radiation therapy. Eur J Surg Oncol, 1999 25(2), 221-222. Weaver, J; Billings, SD. Postradiation cutaneous vascular tumors of the breast: a review. Semin Diagn Pathol, 2009 26(3), 141-149. Abbott, R; Palmieri, C. Angiosarcoma of the breast following surgery and radiotherapy for breast cancer. Nat Clin Pract Oncol, 2008 5(12), 727-736. 230 [343] [344] [345] [346] [347] [348] [349] [350] [351] [352] [353] [354] [355] [356] [357] References Lamblin, G; Oteifa, M; Zinzindohoue, C; Isaac, S; Termine, L; Bobin, JY. Angiosarcoma after conservative treatment and radiation therapy for adenocarcinoma of the breast. Eur J Surg Oncol, 2001 27(2), 146-151. Uğraş, S; Dilek, ON; Karaayvaz, M; Dilek, H; Peker, O; Barut, I. Primary leiomyosarcoma of the breast. Surg Today, 1997 27(11), 1082-1085. Stolnicu, S; Moldovan, C; Podoleanu, C; Georgescu, R. Mesenchymal tumors and tumor-like lesions of the breast: a contemporary approach review. Ann Pathol, 2015 35(1), 15-31. Godwin, Y; McCulloch, TA; Sully, L. Extra-abdominal desmoid tumour of the breast: review of the primary management and the implications for breast reconstruction. Br J Plast Surg, 2001 54(3), 268-271. Hu, Q; Chen, WX; Zhong, SL; Li, J; Luo, Z; Tang, JH; Zhao, JH. Current progress in the treatment of metaplastic breast carcinoma. Asian Pac J Cancer Prev, 2013 14(11), 6221-6225. McKinnon, E; Xiao, P. Metaplastic carcinoma of the breast. Arch Pathol Lab Med, 2015 139(6), 819-822. Iellin, A; Waizbard, E; Levine, T; Behar, A. Malignant fibrous histiocytoma of the breast. Int Surg, 1990 75(1), 63-66. Nicholson, BT; Bhatti, RM; Glassman, L. Extranodal Lymphoma of the Breast. Radiol Clin North Am, 2016 54(4), 711-726. Guerrero, MA; Ballard, BR; Grau, AM. Malignant phyllodes tumor of the breast: review of the literature and case report of stromal overgrowth. Surg Oncol, 2003 12(1), 27-37. Singer, A; Tresley, J; Velazquez-Vega, J; Yepes, M. Unusual aggressive breast cancer: metastatic malignant phyllodes tumor. J Radiol Case Rep, 2013 7(2), 2437. Menes, T; Schachter, J; Morgenstern, S; Fenig, E; Lurie, H; Gutman, H. Primary squamous cell carcinoma (SqCC) of the breast. Am J Clin Oncol, 2003 26(6), 571-573. Sadanaga, N; Okada, S; Shiotani, S; Morita, M; Kakeji, Y; Kitamura, K; Tamiya, S; Sugimachi, K; Maehara, Y. Clinical characteristics of small cell carcinoma of the breast. Oncol Rep, 2008 19(4), 981-985. Mirza, IA; Shahab, N. Small cell carcinoma of the breast. Semin Oncol, 2007 34(1), 64-66. Kobayashi, S. Basal-like subtype of breast cancer: a review of its unique characteristics and their clinical significance. Breast Cancer, 2008 15(2), 153158. Jochems, L; Tjalma, WA. Primary small cell neuroendocrine tumour of the breast. Eur J Obstet Gynecol Reprod Biol, 2004 115(2), 231-233. References [358] [359] [360] [361] [362] [363] [364] [365] [366] [367] [368] [369] [370] [371] [372] 231 Adams, RW; Dyson, P; Barthelmes, L. Neuroendocrine breast tumours: breast cancer or neuroendocrine cancer presenting in the breast? Breast, 2014 23(2), 120-127. Inno, A; Bogina, G; Turazza, M; Bortesi, L; Duranti, S; Massocco, A; Zamboni, G; Carbognin, G; Alongi, F; Salgarello, M; Gori, S. Neuroendocrine Carcinoma of the Breast: Current Evidence and Future Perspectives. Oncologist, 2016 21(1), 28-32. Gutermuth, J; Audring, H; Voit, C; Haas, N. Primary carcinoma of ectopic axillary breast tissue. J Eur Acad Dermatol Venereol, 2006 20(2), 217-221. Visconti, G; Eltahir, Y; Van Ginkel, RJ; Bart, J; Werker, PM. Approach and management of primary ectopic breast carcinoma in the axilla: where are we? A comprehensive historical literature review. J Plast Reconstr Aesthet Surg, 2011 64(1), e1-e11. Bartella, L; Kaye, J; Perry, NM; Malhotra, A; Evans, D; Ryan, D; Wells, C; Vinnicombe, SJ. Metastases to the breast revisited: radiologicalhistopathological correlation. Clin Radiol, 2003 58(7), 524-531. Agnese, DM; Burak, WE Jr. Ablative approaches to the minimally invasive treatment of breast cancer. Cancer J, 2005 11(1), 77-82. Hall-Craggs, MA; Vaidya, JS. Minimally invasive therapy for the treatment of breast tumours. Eur J Radiol, 2002 42(1), 52-57. Lanzafame, RJ. Applications of laser technology in breast cancer therapy. Semin Surg Oncol, 1995 11(4), 328-332. Need, EF; Atashgaran, V; Ingman, WV; Dasari, P. Hormonal regulation of the immune microenvironment in the mammary gland. J Mammary Gland Biol Neoplasia, 2014 19(2), 229-239. Fentiman, IS; Gregory, WM. The hormonal milieu and prognosis in operable breast cancer. Cancer Surv, 1993 18, 149-163. Kroman, N. Timing of breast cancer surgery in relation to the menstrual cycle – The rise and fall of a hypothesis. Acta Oncol, 2008 47(4), 576-579. Singletary, SE. Minimally invasive surgery in breast cancer treatment. Biomed Pharmacother, 2001 55(9-10), 510-514. Luini, A; Gatti, G; Galimberti, V; Zurrida, S; Intra, M; Gentilini, O; Paganelli, G; Viale, G; Orecchia, R; Veronesi, P; Veronesi, U. Conservative treatment of breast cancer: its evolution. Breast Cancer Res Treat, 2005 94(3), 195-198. Forrest, AP. Conservative local treatment of breast cancer. Cancer, 1977 39(6 Suppl), 2813-2821. Fredericks, S. A 10-year experience with subcutaneous mastectomy. Clin Plast Surg, 1975 2(3), 347-357. 232 [373] [374] [375] [376] [377] [378] [379] [380] [381] [382] [383] [384] [385] [386] [387] References Patani, N; Mokbel, K. Oncological and aesthetic considerations of skin-sparing mastectomy. Breast Cancer Res Treat, 2008 111(3), 391-403. Huang, NS; Wu, J. Nipple-sparing Mastectomy in Breast Cancer: From an Oncologic Safety Perspective. Chin Med J (Engl), 2015 128(16), 2256-2261. Sisco, M; Yao, KA. Nipple-sparing mastectomy: A contemporary perspective. J Surg Oncol, 2016 113(8), 883-890. Haloua, MH; Krekel, NM; Winters, HA; Rietveld, DH; Meijer, S; Bloemers, FW; van den Tol, MP. A systematic review of oncoplastic breast-conserving surgery: current weaknesses and future prospects. Ann Surg, 2013 257(4), 609620. Mustonen, P; Härmä, M. Viewpoints on oncoplastic surgery in invasive breast cancer. Scand J Surg, 2002 91(3), 255, 258-262. Chen, CY; Calhoun, KE; Masetti, R; Anderson, BO. Oncoplastic breast conserving surgery: a renaissance of anatomically-based surgical technique. Minerva Chir, 2006 61(5), 421-434. Silverstein, MJ; Mai, T; Savalia, N; Vaince, F; Guerra, L. Oncoplastic breast conservation surgery: the new paradigm. J Surg Oncol, 2014 110(1), 82-89. Clough, KB; Benyahi, D; Nos, C; Charles, C; Sarfati, I. Oncoplastic surgery: pushing the limits of breast-conserving surgery. Breast J, 2015 21(2), 140-146. Weber, WP; Soysal, SD; Fulco, I; Barandun, M; Babst, D; Kalbermatten, D; Schaefer, DJ; Oertli, D; Kappos, EA; Haug, M. Standardization of oncoplastic breast conserving surgery. Eur J Surg Oncol, 2017 43(7), 1236-1243. Barrio, AV; Morrow, M. Appropriate margin for lumpectomy excision of invasive breast cancer. Chin Clin Oncol, 2016 5(3), 35. Krontiras, H; Lancaster, RB; Urist, MM. What is a clear margin in breast conserving cancer surgery? Curr Treat Options Oncol, 2014 15(1), 79-85. Gould, EW; Robinson, PG. The pathologist’s examination of the “lumpectomy” – The pathologists’ view of surgical margins. Semin Surg Oncol, 1992 8(3), 129135. Rubio, IT; Ahmed, M; Kovacs, T; Marco, V. Margins in breast conserving surgery: A practice-changing process. Eur J Surg Oncol, 2016 42(5), 631-640. O’Kelly Priddy, CM; Forte, VA; Lang, JE. The importance of surgical margins in breast cancer. J Surg Oncol, 2016 113(3), 256-263. Galimberti, V; Taffurelli, M; Leonardi, MC; Aristei, C; Trentin, C; Cassano, E; Pietribiasi, F; Corso, G; Munzone, E; Tondini, C; Frigerio, A; Cataliotti, L; Santini, D. Surgical resection margins after breast-conserving surgery: Senonetwork recommendations. Tumori, 2016 2016(3), 284-289. References [388] [389] [390] [391] [392] [393] [394] [395] [396] [397] [398] [399] 233 Ang, SC; Tapia, G; Davidson, EJ; Kahramangil, B; Mak, C; Carmalt, H; Warrier, S. Positive anterior margins in breast conserving surgery: Does it matter? A systematic review of the literature. Breast, 2016 27, 105-108. Reitsamer, R; Sedlmayer, F; Kopp, M; Kametriser, G; Menzel, C; Glueck, S; Nairz, O; Deutschmann, H; Merz, F; Peintinger, F. Concepts and techniques of intraoperative radiotherapy (IORT) for breast cancer. Breast Cancer, 2008 15(1), 40-46. Tobias, JS; Vaidya, JS; Keshtgar, M; Douek, M; Metaxas, M; Stacey, C; Sainsbury, R; D’Souza, D; Baum, M. Breast-conserving surgery with intraoperative radiotherapy: the right approach for the 21st century? Clin Oncol (R Coll Radiol), 2006 18(3), 220-228. Sautter-Bihl, ML; Sedlmayer, F; Budach, W; Dunst, J; Engenhart-Cabillic, R; Fietkau, R; Feyer, P; Haase, W; Harms, W; Rödel, C; Souchon, R; Wenz, F; Sauer, R. Intraoperative radiotherapy as accelerated partial breast irradiation for early breast cancer : beware of one-stop shops? Strahlenther Onkol, 2010 186(12), 651-657. Zurrida, S; Leonardi, MC; Del Castillo, A; Lazzari, R; Arnone, P; Caldarella, P. Accelerated partial breast irradiation in early breast cancer: focus on intraoperative treatment with electrons (ELIOT). Womens Health (Lond), 2012 8(1), 89-98. Trifiletti, DM; Jones, R; Showalter, SL; Libby, BB; Brenin, DR; Schroen, A; Morris, MM; Reardon, KA; Showalter, TN. Techniques for intraoperative radiation therapy for early-stage breast carcinoma. Future Oncol, 2015 11(7), 1047-1058. Merrick, HW 3rd; Hager, E; Dobelbower, RR Jr. Intraoperative radiation therapy for breast cancer. Surg Oncol Clin N Am, 2003 12(4), 1065-1078. Esposito, E; Compagna, R; Rinaldo, M; Falivene, S; Ravo, V; Amato, B; Muto, P; D’Aiuto, M. Intraoperative radiotherapy in elderly patients with breast cancer: Is there a clinical applicability? Review of the current evidence. Int J Surg, 2016 33 Suppl 1, S88-S91. Esposito, E; Anninga, B; Harris, S; Capasso, I; D’Aiuto, M; Rinaldo, M; Douek, M. Intraoperative radiotherapy in early breast cancer. Br J Surg, 2015 102(6), 599-610. Holmes, DR. Intraoperative radiotherapy in breast conserving surgery. J Surg Oncol, 2014 110(1), 68-74. Holmes, DR. Intraoperative radiotherapy of the breast for early-stage breast cancer: ready for primetime. Breast J, 2015 21(2), 181-184. Mendelson, EB. Evaluation of the postoperative breast. Radiol Clin North Am, 1992 30(1), 107-138. 234 [400] [401] [402] [403] [404] [405] [406] [407] [408] [409] [410] [411] [412] [413] [414] [415] [416] References Neal, CH; Yilmaz, ZN; Noroozian, M; Klein, KA; Sundaram, B; Kazerooni, EA; Stojanovska, J. Imaging of breast cancer-related changes after surgical therapy. AJR Am J Roentgenol, 2014 202(2), 262-272. Lagios, MD; Bennington, JL. Protocol for the pathologic examination and tissue processing of the mammographically directed breast biopsy. Pathology (Phila), 1992 1(1), 23-45. de Paredes, ES; Langer, TG; Cousins, J. Interventional breast procedures. Curr Probl Diagn Radiol, 1998 27(5), 133-184. Jesinger, RA. Breast anatomy for the interventionalist. Tech Vasc Interv Radiol, 2014 17(1), 3-9. Newman, J. Role of stereotactic biopsy in diagnosing breast cancer. Radiol Technol, 1996 68(2), 131-148. Burns, RP. Image-guided breast biopsy. Am J Surg, 1997 173(1), 9-11. Harolds, JA. Stereotactically guided needle biopsy of the breast for nonpalpable lesions. J Okla State Med Assoc, 1993 86(12), 604-612. Chilcote, WA; Quinn, CA. Stereotactic breast biopsy: a less-invasive option. Cleve Clin J Med, 1997 64(10), 550-554. Jackson, VP. The status of mammographically guided fine needle aspiration biopsy of nonpalpable breast lesions. Radiol Clin North Am, 1992 30(1), 155166. Dershaw, DD. Stereotaxic breast biopsy. Semin Ultrasound CT MR, 1996;17(5), 444-459. Bernstein, JR. Role of stereotactic breast biopsy. Semin Surg Oncol, 1996 12(5), 290-299. Dershaw, DD; Liberman, L. Stereotactic breast biopsy: indications and results. Oncology (Williston Park), 1998 12(6), 907-916. Fajardo, LL; DeAngelis, GA. The role of stereotactic biopsy in abnormal mammograms. Surg Oncol Clin N Am, 1997 6(2), 285-299. Huang, ML; Adrada, BE; Candelaria, R; Thames, D; Dawson, D; Yang, WT. Stereotactic breast biopsy: pitfalls and pearls. Tech Vasc Interv Radiol, 2014 17(1), 32-39. Wong, AY; Salisbury, E; Bilous, M. Recent developments in stereotactic breast biopsy methodologies: an update for the surgical pathologist. Adv Anat Pathol, 2000 7(1), 26-35. Liberman, L. Clinical management issues in percutaneous core breast biopsy. Radiol Clin North Am, 2000 38(4), 791-807. Symmans, WF; Cangiarella, JF; Gottlieb, S; Newstead, GM; Waisman, J. What is the role of cytopathologists in stereotaxic needle biopsy diagnosis of References [417] [418] [419] [420] [421] [422] [423] [424] [425] [426] [427] [428] [429] [430] [431] [432] 235 nonpalpable mammographic abnormalities? Diagn Cytopathol, 2001 24(4), 260270. Shulman, SG; March, DE. Ultrasound-guided breast interventions: accuracy of biopsy techniques and applications in patient management. Semin Ultrasound CT MR, 2006 27(4), 298-307. Sadowsky, NL; Semine, AA; Levin, E; Kelly, E. Ultrasonographic guidance for needle biopsy of breast lesions. Surg Oncol Clin N Am, 1997 6(2), 265-284. Fornage, BD; Coan, JD; David, CL. Ultrasound-guided needle biopsy of the breast and other interventional procedures. Radiol Clin North Am, 1992 30(1), 167-185. Fornage, BD. Sonographically guided needle biopsy of nonpalpable breast lesions. J Clin Ultrasound, 1999 27(7), 385-398. Henry-Tillman, R; Johnson, AT; Smith, LF; Klimberg, VS. Intraoperative ultrasound and other techniques to achieve negative margins. Semin Surg Oncol, 2001 20(3), 206-213. Raghu, M; Hooley, R. Breast ultrasound for the interventionalist. Tech Vasc Interv Radiol, 2014 17(1), 16-22. Lieu, D. Breast imaging for interventional pathologists. Arch Pathol Lab Med, 2013 137(1), 100-119. Fornage, BD; Sneige, N; Edeiken, BS. Interventional breast sonography. Eur J Radiol, 2002 42(1), 17-31. Youk, JH; Kim, EK; Kim, MJ; Lee, JY; Oh, KK. Missed breast cancers at USguided core needle biopsy: how to reduce them. Radiographics, 2007 27(1), 7994. Klimberg, VS; Rivere, A. Ultrasound image-guided core biopsy of the breast. Chin Clin Oncol, 2016 5(3), 33. Ahmed, M; Abdullah, N; Cawthorn, S; Usiskin, SI; Douek, M. Why should breast surgeons use ultrasound? Breast Cancer Res Treat, 2014 145(1), 1-4. van den Bosch, MA; Daniel, BL. MR-guided interventions of the breast. Magn Reson Imaging Clin N Am, 2005 13(3), 505-517. Daniel, BL. Intraprocedural magnetic resonance imaging-guided interventions in the breast. Top Magn Reson Imaging, 2000 11(3), 184-190. King, TA; Fuhrman, GM. Image-guided breast biopsy. Semin Surg Oncol, 2001 20(3), 197-204. Lo, LD; Orel, SG; Schnall, MD. MR imaging-guided interventions in the breast. Magn Reson Imaging Clin N Am, 2001 9(2), 373-380. Harms, SE. MR-guided minimally invasive procedures. Magn Reson Imaging Clin N Am, 2001 9(2), 381-392. 236 [433] [434] [435] [436] [437] [438] [439] [440] [441] [442] [443] [444] [445] [446] [447] References Helbich, TH. Localization and biopsy of breast lesions by magnetic resonance imaging guidance. J Magn Reson Imaging, 2001 13(6), 903-911. Eby, PR; Lehman, C. MRI-guided breast interventions. Semin Ultrasound CT MR, 2006 27(4), 339-350. Floery, D; Helbich, TH. MRI-Guided percutaneous biopsy of breast lesions: materials, techniques, success rates, and management in patients with suspected radiologic-pathologic mismatch. Magn Reson Imaging Clin N Am, 2006 14(3), 411-425. Kaiser, WA; Pfleiderer, SO; Baltzer, PA. MRI-guided interventions of the breast. J Magn Reson Imaging, 2008 27(2), 347-355. Eby, PR; Lehman, CD. Magnetic resonance imaging-guided breast interventions. Top Magn Reson Imaging, 2008 19(3), 151-162. Postma, EL; van Hillegersberg, R; Daniel, BL; Merckel, LG; Verkooijen, HM; van den Bosch, MA. MRI-guided ablation of breast cancer: where do we stand today? J Magn Reson Imaging, 2011 34(2), 254-261. Price, ER. Magnetic resonance imaging-guided biopsy of the breast: fundamentals and finer points. Magn Reson Imaging Clin N Am, 2013 21(3), 571-581. Chevrier, MC; David, J; Khoury, ME; Lalonde, L; Labelle, M; Trop, I. Breast Biopsies Under Magnetic Resonance Imaging Guidance: Challenges of an Essential but Imperfect Technique. Curr Probl Diagn Radiol, 2016 45(3), 193204. Maglione, KD; Lee, AY; Ray, KM; Joe, BN; Balassanian, R. RadiologicPathologic Correlation for Benign Results After MRI-Guided Breast Biopsy. AJR Am J Roentgenol, 2017 209(2), 442-453. Viehweg, P; Heinig, A; Amaya, B; Alberich, T; Laniado, M, HeywangKöbrunner SH. MR-guided interventional breast procedures considering vacuum biopsy in particular. Eur J Radiol. 2002 Apr;42(1):32-9. Gombos, EC; Jagadeesan, J; Richman, DM; Kacher, DF. Magnetic Resonance Imaging-Guided Breast Interventions: Role in Biopsy Targeting and Lumpectomies. Magn Reson Imaging Clin N Am, 2015 23(4), 547-561. Hoorntje, LE; Peeters, PH; Mali, WP; Borel Rinkes, IH. Vacuum-assisted breast biopsy: a critical review. Eur J Cancer, 2003 39(12), 1676-1683. Plantade, R; Thomassin-Naggara, I. MRI vacuum-assisted breast biopsies. Diagn Interv Imaging, 2014 95(9), 779-801. Iwuagwu, O; Drew, P. Vacuum-assisted biopsy device-diagnostic and therapeutic applications in breast surgery. Breast, 2004 13(6), 483-487. Cusumano, P; Polkowski, WP; Liu, H; Schulz-Wendtland, R; Janssens, J. Percutaneous tissue acquisition: a treatment for breast cancer? Vacuum-assisted References [448] [449] [450] [451] [452] [453] [454] [455] [456] [457] [458] [459] [460] [461] 237 biopsy devices are not indicated for extended tissue removal. Eur J Cancer Prev, 2008 17(4), 323-330. Boppart, SA; Luo, W; Marks, DL; Singletary, KW. Optical coherence tomography: feasibility for basic research and image-guided surgery of breast cancer. Breast Cancer Res Treat, 2004 84(2), 85-97. Tomkovich, KR. Breast interventions: a primer for interventional radiologists. Tech Vasc Interv Radiol, 2006 9(1), 30-35. Kacher, DF; Jolesz, FA. MR imaging-guided breast ablative therapy. Radiol Clin North Am, 2004 42(5), 947-962. Fornage, BD; Hwang, RF. Current status of imaging-guided percutaneous ablation of breast cancer. AJR Am J Roentgenol, 2014 203(2), 442-448. Roubidoux, MA; Yang, W; Stafford, RJ. Image-guided ablation in breast cancer treatment. Tech Vasc Interv Radiol, 2014 17(1), 49-54. Lanza, E; Palussiere, J; Buy, X; Grasso, RF; Beomonte Zobel, B; Poretti, D; Pedicini, V; Balzarini, L; Cazzato, RL. Percutaneous Image-Guided Cryoablation of Breast Cancer: A Systematic Review. J Vasc Interv Radiol, 2015 26(11), 1652-1657.e1. Fleming, MM; Holbrook, AI; Newell, MS. Update on Image-Guided Percutaneous Ablation of Breast Cancer. AJR Am J Roentgenol, 2017 208(2), 267-274. Laucirica, R. Intraoperative assessment of the breast: guidelines and potential pitfalls. Arch Pathol Lab Med, 2005 129(12), 1565-1574. Bigio, IJ. Real-time pathology to guide breast surgery: seeing alone is not believing. Clin Cancer Res, 2012 18(22), 6083-6085. Thill, M. MarginProbe: intraoperative margin assessment during breast conserving surgery by using radiofrequency spectroscopy. Expert Rev Med Devices, 2013 10(3), 301-315. Thill, M; Baumann, K; Barinoff, J. Intraoperative assessment of margins in breast conservative surgery – Still in use? J Surg Oncol, 2014 110(1), 15-20. Hargreaves, AC; Mohamed, M; Audisio, RA. Intra-operative guidance: methods for achieving negative margins in breast conserving surgery. J Surg Oncol, 2014 110(1), 21-25. Butler-Henderson, K; Lee, AH; Price, RI; Waring, K. Intraoperative assessment of margins in breast conserving therapy: a systematic review. Breast, 2014 23(2), 112-119. Aydogan, F; Velidedeoglu, M; Kilic, F; Yilmaz, H. Radio-guided localization of clinically occult breast lesions: current modalities and future directions. Expert Rev Med Devices, 2014 11(1), 53-63. 238 [462] [463] [464] [465] [466] [467] [468] [469] [470] [471] [472] References Langhans, L; Klausen, TL; Tvedskov, TF; Vejborg, I; Kroman, N; Hesse, B. Radioguided Surgery for Localization of Nonpalpable Breast Lesions A MiniReview. Curr Radiopharm, 2016 9(2), 114-120. van der Ploeg, IM; Hobbelink, M; van den Bosch, MA; Mali, WP; Borel Rinkes, IH; van Hillegersberg, R. “Radioguided occult lesion localization” (ROLL) for non-palpable breast lesions: a review of the relevant literature. Eur J Surg Oncol, 2008 34(1), 1-5. Rovera, F; Frattini, F; Marelli, M; Corben, AD; Vanoli, C; Dionigi, G; Boni, L; Dionigi, R. Radio-guided occult lesion localization versus wire-guided localization in non-palpable breast lesions. Int J Surg, 2008 6 Suppl 1, S101S103. McClatchy, DM 3rd; Krishnaswamy, V; Kanick, SC; Elliott, JT; Wells, WA; Barth, RJ Jr; Paulsen, KD; Pogue, BW. Molecular dyes used for surgical specimen margin orientation allow for intraoperative optical assessment during breast conserving surgery. J Biomed Opt, 2015 20(4), 040504. Miller-Kleinhenz, JM; Bozeman, EN; Yang, L. Targeted nanoparticles for image-guided treatment of triple-negative breast cancer: clinical significance and technological advances. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 2015 7(6), 797-816. St John, ER; Al-Khudairi, R; Ashrafian, H; Athanasiou, T; Takats, Z; Hadjiminas, DJ; Darzi, A; Leff, DR. Diagnostic Accuracy of Intraoperative Techniques for Margin Assessment in Breast Cancer Surgery: A Meta-analysis. Ann Surg, 2017 265(2), 300-310. Hazard, HW; Hansen, NM. Image-guided procedures for breast masses. Adv Surg, 2007 41: 257-272. Wallis, M; Tardivon, A; Helbich, T; Schreer, I; European Society of Breast Imaging. Guidelines from the European Society of Breast Imaging for diagnostic interventional breast procedures. Eur Radiol, 2007 17(2), 581-588. Smetherman, DH. Screening, imaging, and image-guided biopsy techniques for breast cancer. Surg Clin North Am, 2013 93(2), 309-327. Masood, S; Rosa, M; Kraemer, DF; Smotherman, C; Mohammadi, A. Comparative cost-effectiveness of fine needle aspiration biopsy versus imageguided biopsy, and open surgical biopsy in the evaluation of breast cancer in the era of Affordable Care Act: a changing landscape. Diagn Cytopathol, 2015 43(8), 605-612. Görkem, SB; O’Connell, AM. Abnormal axillary lymph nodes on negative mammograms: causes other than breast cancer. Diagn Interv Radiol, 2012;18(5), 473-479. References [473] [474] [475] [476] [477] [478] [479] [480] [481] [482] [483] [484] [485] [486] 239 Querci Della Rovere, G; Benson, JR. A critique of the sentinel node concept. Breast, 2006 15(6), 693-697. Ung, O; Tan, M; Chua, B; Barraclough, B. Complete axillary dissection: a technique that still has relevance in contemporary management of breast cancer. ANZ J Surg, 2006 76(6), 518-521. Mansel, RE; Khonji, NI; Clarke, D. History, present status and future of sentinel node biopsy in breast cancer. The Mary Béves Lecture. Acta Oncol, 2000 39(3), 265-268. Mariani, G; Moresco, L; Viale, G; Villa, G; Bagnasco, M; Canavese, G; Buscombe, J; Strauss, HW; Paganelli, G. Radioguided sentinel lymph node biopsy in breast cancer surgery. J Nucl Med, 2001 42(8), 1198-1215. Nathanson, SD. Insights into the mechanisms of lymph node metastasis. Cancer, 2003 98(2), 413-423. Amin, BD; Hoda, SA. Minimal metastatic disease in sentinel lymph nodes in breast carcinoma: some modest proposals to refine criteria for “isolated tumor cells.” Adv Anat Pathol, 2006 13(4), 185-189. Tsuda, H. Histological examination of sentinel lymph nodes: significance of macrometastasis, micrometastasis, and isolated tumor cells. Breast Cancer, 2015 22(3), 221-229. Douglas-Jones, AG; Woods, V. Molecular assessment of sentinel lymph node in breast cancer management. Histopathology, 2009 55(1), 107-113. Cserni, G. Minimal disease in sentinel nodes. Pathol Oncol Res, 2008 14(2), 117-121. Tanis, PJ; Nieweg, OE; Valdés Olmos, RA; Kroon, BB. Anatomy and physiology of lymphatic drainage of the breast from the perspective of sentinel node biopsy. J Am Coll Surg, 2001 192(3), 399-409. Tanis, PJ; van Rijk, MC; Nieweg, OE. The posterior lymphatic network of the breast rediscovered. J Surg Oncol, 2005 91(3), 195-198. Diaz, NM; Vrcel, V; Centeno, BA; Muro-Cacho, C. Modes of benign mechanical transport of breast epithelial cells to axillary lymph nodes. Adv Anat Pathol, 2005 12(1), 7-9. Papaioannou, A. The contribution of regional lymph nodes in the resistance against breast cancer: practical implications. J Surg Oncol, 1984 25(4), 232-239. Alazraki, NP; Styblo, T; Grant, SF; Cohen, C; Larsen, T; Waldrop, S; Aarsvold, JN. Sentinel node staging of early breast cancer using lymphoscintigraphy and the intraoperative gamma detecting probe. Radiol Clin North Am, 2001 39(5), 947-956. 240 [487] [488] [489] [490] [491] [492] [493] [494] [495] [496] [497] [498] [499] [500] [501] [502] References Blichert-Toft, M. Axillary surgery in breast cancer management – Background, incidence and extent of nodal spread, extent of surgery and accurate axillary staging, surgical procedures. Acta Oncol, 2000 39(3), 269-275. Euhus, DM. Cytokeratin staining and other sentinel node controversies. Clin Breast Cancer, 2003 4 Suppl 1, S49-S54. Manca, G; Volterrani, D; Mazzarri, S; Duce, V; Svirydenka, A; Giuliano, A; Mariani, G. Sentinel lymph node mapping in breast cancer: a critical reappraisal of the internal mammary chain issue. Q J Nucl Med Mol Imaging, 2014 58(2), 114-126. Chen, RC; Lin, NU; Golshan, M; Harris, JR; Bellon, JR. Internal mammary nodes in breast cancer: diagnosis and implications for patient management – A systematic review. J Clin Oncol, 2008 26(30), 4981-4989. Abdullgaffar, B; Gopal, P; Abdulrahim, M; Ghazi, E; Mohamed, E. The significance of intramammary lymph nodes in breast cancer: a systematic review and meta-analysis. Int J Surg Pathol, 2012 20(6), 555-563. Morrow, M; Foster, RS Jr. Staging of breast cancer: a new rationale for internal mammary node biopsy. Arch Surg, 1981 116(6), 748-751. Bembenek, A; Schlag, PM. Lymph-node dissection in breast cancer. Langenbecks Arch Surg, 2000 385(4), 236-245. Sacre, R. Modern thoughts on lymph nodes in breast cancer. Semin Surg Oncol, 1989 5(2), 118-125. Noguchi, M. Relevance and practicability of internal mammary sentinel node biopsy for breast cancer. Breast Cancer, 2002 9(4), 329-336. Bevilacqua, JL; Gucciardo, G; Cody, HS; MacDonald, KA; Sacchini, V; Borgen, PI; Van Zee, KJ. A selection algorithm for internal mammary sentinel lymph node biopsy in breast cancer. Eur J Surg Oncol, 2002 28(6), 603-614. Cserni, G; Szekeres, JP. Internal mammary lymph nodes and sentinel node biopsy in breast cancer. Surg Oncol, 2001 10(1-2), 25-33. Jatoi, I. Internal mammary sentinel nodes in primary breast cancer. Curr Med Res Opin, 2003 19(6), 567-569. Crowe, P; Temple, W. Management of the axilla in early breast cancer: is it time to change tack? Aust N Z J Surg, 2000 70(4), 288-296. Epstein, RJ. Routine or delayed axillary dissection for primary breast cancer? Eur J Cancer, 1995 31A(10), 1570-1573. Noguchi, M; Katev, N; Miyazaki, I. Diagnosis of axillary lymph node metastases in patients with breast cancer. Breast Cancer Res Treat, 1996 40(3), 283-293. Bombardieri, E; Crippa, F; Maffioli, L; Draisma, A; Chiti, A; Agresti, R; Greco, M. Nuclear medicine approaches for detection of axillary lymph node metastases. Q J Nucl Med, 1998 42(1), 54-65. References [503] [504] [505] [506] [507] [508] [509] [510] [511] [512] [513] [514] [515] [516] 241 Crippa, F; Gerali, A; Alessi, A; Agresti, R; Bombardieri, E. FDG-PET for axillary lymph node staging in primary breast cancer. Eur J Nucl Med Mol Imaging, 2004 31 Suppl 1, S97-S102. Noguchi, M. Sentinel lymph node biopsy as an alternative to routine axillary lymph node dissection in breast cancer patients. J Surg Oncol, 2001 76(2), 144156. Goyal, A; Mansel, RE. Recent advances in sentinel lymph node biopsy for breast cancer. Curr Opin Oncol, 2008 20(6), 621-626. Cools-Lartigue, J; Meterissian, S. Accuracy of axillary ultrasound in the diagnosis of nodal metastasis in invasive breast cancer: a review. World J Surg, 2012 36(1), 46-54. Ahmed, M; Usiskin, SI; Hall-Craggs, MA; Douek, M. Is imaging the future of axillary staging in breast cancer? Eur Radiol, 2014 24(2), 288-293. Motomura, K. Sentinel node biopsy for breast cancer: past, present, and future. Breast Cancer, 2015 22(3), 212-220. Creager, AJ; Geisinger, KR. Intraoperative evaluation of sentinel lymph nodes for breast carcinoma: current methodologies. Adv Anat Pathol, 2002 9(4), 233243. Hakam, A; Khin, NN. Intraoperative imprint cytology in assessment of sentinel lymph nodes and lumpectomy surgical margins. Clin Lab Med, 2005 25(4), 795807. Aubard, Y; Mollard, J; Fermeaux, V. How to avoid the uncertainties of intraoperative examination of the sentinel lymph node in breast cancer? Eur J Gynaecol Oncol, 2003 24(5), 357-359. Nieweg, OE; Estourgie, SH; Valdés Olmos, RA; Rutgers, EJ; Hoefnagel, CA; Kroon, BB. Lymphatic mapping with tracer administration into the primary breast cancer. Eur J Surg Oncol, 2003 29(1), 95-97. Masannat, Y; Shenoy, H; Speirs, V; Hanby, A; Horgan, K. Properties and characteristics of the dyes injected to assist axillary sentinel node localization in breast surgery. Eur J Surg Oncol, 2006 32(4), 381-384. Thevarajah, S; Huston, TL; Simmons, RM. A comparison of the adverse reactions associated with isosulfan blue versus methylene blue dye in sentinel lymph node biopsy for breast cancer. Am J Surg, 2005 189(2), 236-239. Peek, MC; Charalampoudis, P; Anninga, B; Baker, R; Douek, M. Blue dye for identification of sentinel nodes in breast cancer and malignant melanoma: a systematic review and meta-analysis. Future Oncol, 2017 13(5), 455-467. Li, J; Zhuang, Z; Jiang, B; Zhao, P; Lin, C. Advances and perspectives in nanoprobes for noninvasive lymph node mapping. Nanomedicine (Lond), 2015 10(6), 1019-1036. 242 [517] [518] [519] [520] [521] [522] [523] [524] [525] [526] [527] [528] [529] [530] References Meyer, JS. Sentinel lymph node biopsy: strategies for pathologic examination of the specimen. J Surg Oncol, 1998 69(4), 212-218. Kiricuta, IC. Sentinel node concept in breast cancer. Strahlenther Onkol, 2000 176(7), 307-314. Tjan-Heijnen, VC; Buit, P; de Widt-Evert, LM; Ruers, TJ; Beex, LV. Micrometastases in axillary lymph nodes: an increasing classification and treatment dilemma in breast cancer due to the introduction of the sentinel lymph node procedure. Breast Cancer Res Treat, 2001 70(2), 81-88. Weaver, DL. Pathology evaluation of sentinel lymph nodes in breast cancer: protocol recommendations and rationale. Mod Pathol, 2010 23 Suppl 2, S26S32. Cserni, G. Histopathologic examination of the sentinel lymph nodes. Breast J, 2006 12(5 Suppl 2), S152-S156. Glass, EC; Essner, R; Giuliano, AE. Sentinel node localization in breast cancer. Semin Nucl Med, 1999 29(1), 57-68. Cody, HS 3rd. Sentinel lymph node mapping in breast cancer. Oncology (Williston Park), 1999 13(1), 25-34. Cox, CE; Haddad, F; Bass, S; Cox, JM; Ku, NN; Berman, C; Shons, AR; Yeatman, T; Pendas, S; Reintgen, DS. Lymphatic mapping in the treatment of breast cancer. Oncology (Williston Park), 1998 12(9), 1283-1292. McIntosh, SA; Purushotham, AD. Lymphatic mapping and sentinel node biopsy in breast cancer. Br J Surg, 1998 85(10), 1347-1356. Goyal, A; Mansel, RE. Does imaging in sentinel node scintigraphic localization add value to the procedure in patients with breast cancer? Nucl Med Commun, 2005 26(10), 845-847. Fentiman, IS. Is axillary clearance the standard of care for breast cancer patients with sentinel node involvement? Future Oncol, 2006 2(5), 621-626. Unal, B; Gur, AS; Kayiran, O; Johnson, R; Ahrendt, G; Bonaventura, M; Soran, A. Models for predicting non-sentinel lymph node positivity in sentinel node positive breast cancer: the importance of scoring system. Int J Clin Pract, 2008 62(11), 1785-1791. Schwartz, GF. Clinical practice guidelines for the use of axillary sentinel lymph node biopsy in carcinoma of the breast: current update. Breast J, 2004 10(2), 8588. Hindié, E; Groheux, D; Brenot-Rossi, I; Rubello, D; Moretti, JL; Espié, M. The sentinel node procedure in breast cancer: nuclear medicine as the starting point. J Nucl Med, 2011 52(3), 405-414. References [531] [532] [533] [534] [535] [536] [537] [538] [539] [540] [541] [542] [543] [544] [545] 243 Alazraki, NP; Styblo, T; Grant, SF; Cohen, C; Larsen, T; Aarsvold, JN. Sentinel node staging of early breast cancer using lymphoscintigraphy and the intraoperative gamma-detecting probe. Semin Nucl Med, 2000 30(1), 56-64. Ahmed, M. Sentinel lymph node identification rates and axillary concordance can only be accurately determined by comparing “like with like” injected materials. Breast Cancer Res Treat, 2014 146(1), 229-230. Leong, SP; Morita, ET; Treseler, PA; Wong, JH. Multidisciplinary approach to selective sentinel lymph node mapping in breast cancer. Breast Cancer, 2000 7(2), 105-113. Bonnema, J; van de Velde, CJ. Sentinel lymph node biopsy in breast cancer. Ann Oncol, 2002 13(10), 1531-1537. Allweis, TM; Badriyyah, M; Bar Ad, V; Cohen, T; Freund, HR. Current controversies in sentinel lymph node biopsy for breast cancer. Breast, 2003 12(3), 163-171. Celliers, L; Mann, GB. Alternative sites of injection for sentinel lymph node biopsy in breast cancer. ANZ J Surg, 2003 73(8), 600-604. Noguchi, M. Current controversies concerning sentinel lymph node biopsy for breast cancer. Breast Cancer Res Treat, 2004 84(3), 261-271. Lindahl, T; Engel, G; Ahlgren, J; Klaar, S; Bjöhle, J; Lindman, H; Andersson, J; von Schoultz, E; Bergh, J. Can axillary dissection be avoided by improved molecular biological diagnosis? Acta Oncol, 2000 39(3), 319-326. Terribile, D; Palumbo, F; Nardone, L. Prognostic role of sentinel lymph node biopsy in breast cancer. Rays, 2002 27(4), 291-294. van der Ploeg, IM; Valdés Olmos, RA; Kroon, BB; Nieweg, OE. The Hybrid SPECT/CT as an additional lymphatic mapping tool in patients with breast cancer. World J Surg, 2008 32(9), 1930-1934. Vercellino, L; Ohnona, J; Groheux, D; Slama, A; Colletti, PM; Chondrogiannis, S; Merlet, P; Toubert, ME; Rubello, D. Role of SPECT/CT in sentinel lymph node detection in patients with breast cancer. Clin Nucl Med, 2014 39(5), 431436. Cserni, G. Intraoperative analysis of sentinel lymph nodes in breast cancer by one-step nucleic acid amplification. J Clin Pathol, 2012 65(3), 193-199. Tamaki, Y. One-step nucleic acid amplification assay (OSNA) for sentinel lymph node biopsy. Breast Cancer, 2015 22(3), 230-234. Gervasoni, JE Jr; Taneja, C; Chung, MA; Cady, B. Axillary dissection in the context of the biology of lymph node metastases. Am J Surg, 2000 180(4), 278283. Cody, HS 3rd. Clinical aspects of sentinel node biopsy. Breast Cancer Res, 2001 3(2), 104-108. 244 [546] [547] [548] [549] [550] [551] [552] [553] [554] [555] [556] [557] [558] [559] References Tanis, PJ; Nieweg, OE; Valdés Olmos, RA; Th Rutgers, EJ; Kroon, BB. History of sentinel node and validation of the technique. Breast Cancer Res, 2001 3(2), 109-112. Tuttle, TM. Sentinel lymph node biopsy. Preferred method of axillary staging for breast cancer. Minerva Ginecol, 2005 57(3), 293-303. Bergkvist, L. Resolving the controversies surrounding lymphatic mapping in breast cancer. Future Oncol, 2008 4(5), 681-688. Keshtgar, MR; Baum, M. Axillary dissection over the years: where to from here? World J Surg, 2001 25(6), 761-766. Leong, SP. Paradigm shift of staging and treatment for early breast cancer in the sentinel lymph node era. Breast J, 2006 12(5 Suppl 2):, S128-S133. Ozmen, V; Cabioglu, N. Sentinel lymph node biopsy for breast cancer: current controversies. Breast J, 2006 12(5 Suppl 2), S134-S142. Urban Cde, A; de Lima, RS; Júnior, ES; Neto, CA; Bardoe, SA. Ethics in sentinel node biopsy in breast cancer: an open question. Breast J, 2002 8(4), 253-257. Lyman, GH; Temin, S; Edge, SB; Newman, LA; Turner, RR; Weaver, DL; Benson, AB 3rd; Bosserman, LD; Burstein, HJ; Cody, H 3rd; Hayman, J; Perkins, CL; Podoloff, DA; Giuliano, AE; American Society of Clinical Oncology Clinical Practice. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol, 2014 32(13), 1365-1383. Cantin, J; Scarth, H; Levine, M; Hugi, M; Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Clinical practice guidelines for the care and treatment of breast cancer: 13. Sentinel lymph node biopsy. CMAJ, 2001 165(2), 166-173. Lawn, AM; Frampton, AE; Krell, J; Waheed, S; Stacey-Clear, A. Lymph node ratio can further stratify prognosis in subpopulations of breast cancer patients with axillary nodal metastases. Future Oncol, 2013 9(10), 1425-1431. Ikeda, T. Re-sentinel node biopsy after previous breast and axillary surgery. Surg Today, 2014 44(11), 2015-2021. Pilewskie, M; Morrow, M. Axillary Nodal Management Following Neoadjuvant Chemotherapy: A Review. JAMA Oncol, 2017 3(4), 549-555. Thomson, DR; Trevatt, AE; Furniss, D. When should axillary drains be removed? A meta-analysis of time-limited versus volume controlled strategies for timing of drain removal following axillary lymphadenectomy. J Plast Reconstr Aesthet Surg, 2016 69(12), 1614-1620. Huang, TW; Kuo, KN; Chen, KH; Chen, C; Hou, WH; Lee, WH; Chao, TY; Tsai, JT; Su, CM; Huang, MT; Tam, KW. Recommendation for axillary lymph References [560] [561] [562] [563] [564] [565] [566] [567] [568] [569] [570] [571] [572] 245 node dissection in women with early breast cancer and sentinel node metastasis: A systematic review and meta-analysis of randomized controlled trials using the GRADE system. Int J Surg, 2016 34, 73-80. Jatoi, I; Benson, JR; Toi, M. De-escalation of axillary surgery in early breast cancer. Lancet Oncol, 2016 17(10), e430-e441. Ponzone, R; Ruatta, F; Gatti, M; Castellano, I; Geuna, E; Amato, G; Kubatzki, F; Sgandurra, P; Sapino, A; Montemurro, F. Omission of axillary dissection after a positive sentinel lymph-node: Implications in the multidisciplinary treatment of operable breast cancer. Cancer Treat Rev, 2016 48, 1-7. Reintgen, M; Kerivan, L; Reintgen, E; Swaninathan, S; Reintgen, D. Breast Lymphatic Mapping and Sentinel Lymph Node Biopsy: State of the Art: 2015. Clin Breast Cancer, 2016 16(3), 155-165. Yu, YH; Mo, QG; Zhu, X; Gao, LQ; Liang, C; Huang, Z; Qin, QH; Wei, W; Jiang, Y; Bu, KP; Wei, CY. Axillary fine needle aspiration cytology is a sensitive and highly specific technique for the detection of axillary lymph node metastasis: a meta-analysis and systematic review. Cytopathology, 2016 27(1), 59-69. Maguire, A; Brogi, E. Sentinel lymph nodes for breast carcinoma: an update on current practice. Histopathology, 2016 68(1), 152-167. Wong, JS; Warren, LE; Bellon, JR. Management of the Regional Lymph Nodes in Early-Stage Breast Cancer. Semin Radiat Oncol, 2016 26(1), 37-44. Leff, DR; Vashisht, R; Yongue, G; Keshtgar, M; Yang, GZ; Darzi, A. Endoscopic breast surgery: where are we now and what might the future hold for video-assisted breast surgery? Breast Cancer Res Treat, 2011 125(3), 607-625. Serra-Renom, JM; Guisantes, E; Yoon, T; Benito-Ruiz, J. Endoscopic breast reconstruction with intraoperative complete tissue expansion and partial detachment of the pectoralis muscle. Ann Plast Surg, 2007 58(2), 126-130. Tamaki, Y; Tsukamoto, F; Miyoshi, Y; Tanji, Y; Taguchi, T; Noguchi, S. Overview: video-assisted breast surgery. Biomed Pharmacother, 2002 56 Suppl 1, 187s-191s. Colon, GA. Mammoscopy and endoscopic implant and breast tissue evaluation. Clin Plast Surg, 1995 22(4), 697-706. Faria-Correa, MA. Endoscopic abdominoplasty, mastopexy, and breast reduction. Clin Plast Surg, 1995 22(4), 723-745. Khan SA, Baird, C; Staradub, VL; Morrow, M. Ductal lavage and ductoscopy: the opportunities and the limitations. Clin Breast Cancer, 2002 3(3), 185-191. Kapenhas-Valdes, E; Feldman, SM; Boolbol, SK. The role of mammary ductoscopy in breast cancer: a review of the literature. Ann Surg Oncol, 2008 15(12), 3350-3360. 246 [573] [574] [575] [576] [577] [578] [579] [580] [581] [582] [583] [584] [585] [586] [587] [588] [589] References Mokbel, K; Elkak, AE. The evolving role of mammary ductoscopy. Curr Med Res Opin, 2002 18(1), 30-32. Yamamoto, D; Tanaka, K. A review of mammary ductoscopy in breast cancer. Breast J, 2004 10(4), 295-297. Leris, C; Mokbel, K. The role of mammary ductoscopy in the assessment of breast disease. Int J Fertil Womens Med, 2004 49(5), 200-202. Pereira, B; Mokbel, K. Mammary ductoscopy: past, present, and future. Int J Clin Oncol, 2005 10(2), 112-116. Sauter, E. Breast cancer detection using mammary ductoscopy. Future Oncol, 2005 1(3), 385-393. Sarakbi, WA; Escobar, PF; Mokbel, K. The potential role of breast ductoscopy in breast cancer screening. Int J Fertil Womens Med, 2005 50(5 Pt 1), 208-211. Dooley, WC. The future prospect: ductoscopy-directed brushing and biopsy. Clin Lab Med, 2005 25(4), 845-850. Escobar, PF; Crowe, JP; Matsunaga, T; Mokbel, K. The clinical applications of mammary ductoscopy. Am J Surg, 2006 191(2), 211-215. Jacobs, VR; Paepke, S; Ohlinger, R; Grunwald, S; Kiechle-Bahat, M. Breast ductoscopy: technical development from a diagnostic to an interventional procedure and its future perspective. Onkologie, 2007 30(11), 545-549. Tang, SS; Twelves, DJ; Isacke, CM; Gui, GP. Mammary ductoscopy in the current management of breast disease. Surg Endosc, 2011 25(6), 1712-1722. Eaves, FF 3rd; Bostwick, J 3rd; Nahai, F; Murray, DR; Styblo, TM; Carlson, GW. Endoscopic techniques in aesthetic breast surgery. Augmentation, mastectomy, biopsy, capsulotomy, capsulorrhaphy, reduction, mastopexy, and reconstructive techniques. Clin Plast Surg, 1995 22(4), 683-695. Bilimoria, MM; Morrow, M. The woman at increased risk for breast cancer: evaluation and management strategies. CA Cancer J Clin, 1995 45(5), 263-278. Morrow, M. Identification and management of the woman at increased risk for breast cancer development. Breast Cancer Res Treat, 1994 31(1), 53-60. Stefanek, ME. Bilateral prophylactic mastectomy: issues and concerns. J Natl Cancer Inst Monogr, 1995 (17), 37-42. Radford, DM; Zehnbauer, BA. Inherited breast cancer. Surg Clin North Am, 1996 76(2), 205-220. Kwong, A; Chen, JW; Shin, VY. A new paradigm of genetic testing for hereditary breast/ovarian cancers. Hong Kong Med J, 2016 22(2), 171-177. Kuschel, B; Lux, MP; Goecke, TO; Beckmann, MW. Prevention and therapy for BRCA1/2 mutation carriers and women at high risk for breast and ovarian cancer. Eur J Cancer Prev, 2000 9(3), 139-150. References [590] [591] [592] [593] [594] [595] [596] [597] [598] [599] [600] [601] [602] [603] [604] [605] 247 Mann, GB; Borgen, PI. Breast cancer genes and the surgeon. J Surg Oncol, 1998 67(4), 267-274. Bradbury, AR; Olopade, OI. Genetic susceptibility to breast cancer. Rev Endocr Metab Disord, 2007 8(3), 255-267. Srivastava, A; McKinnon, W; Wood, ME. Risk of breast and ovarian cancer in women with strong family histories. Oncology (Williston Park), 2001 15(7), 889902. Chang-Claude, J. Inherited genetic susceptibility to breast cancer. IARC Sci Publ, 2001 154, 177-190. Webb, M. Developing functional assays for BRCA1 unclassified variants. Methods Mol Biol, 2010 653, 281-291. Yang, Q; Yoshimura, G; Nakamura, M; Nakamura, Y; Suzuma, T; Umemura, T; Mori, I; Sakurai, T; Kakudo, K. BRCA1 in non-inherited breast carcinomas (Review). Oncol Rep, 2002 9(6), 1329-1333. Wicha, MS. Cancer stem cell heterogeneity in hereditary breast cancer. Breast Cancer Res, 2008 10(2), 105. Miyoshi, Y; Murase, K; Oh, K. Basal-like subtype and BRCA1 dysfunction in breast cancers. Int J Clin Oncol, 2008 13(5), 395-400. Ziogas, D; Roukos, DH. Genetics and personal genomics for personalized breast cancer surgery: progress and challenges in research and clinical practice. Ann Surg Oncol, 2009 16(7), 1771-1782. Edlich, RF; Cross, CL; Wack, CA; Chase, ME; Lin, KY; Long, WB 3rd. Breast cancer and ovarian cancer genetics: an update. J Environ Pathol Toxicol Oncol, 2008 27(4), 245-256. Sakorafas, GH; Tsiotou, AG. Genetic predisposition to breast cancer: a surgical perspective. Br J Surg, 2000 87(2), 149-162. Bennett, IC; Gattas, M; Teh, BT. The genetic basis of breast cancer and its clinical implications. Aust N Z J Surg, 1999 69(2), 95-105. Wuttke, M; Phillips, KA. Clinical management of women at high risk of breast cancer. Curr Opin Obstet Gynecol, 2015 27(1), 6-13. Moyer, VA; U.S. Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med, 2014 160(4), 271-281. Wapnir, IL; Rabinowitz, B; Greco, RS. A reappraisal of prophylactic mastectomy. Surg Gynecol Obstet, 1990 171(2), 171-184. Razdan, SN; Patel, V; Jewell, S; McCarthy, CM. Quality of life among patients after bilateral prophylactic mastectomy: a systematic review of patient-reported outcomes. Qual Life Res, 2016 25(6), 1409-1421. 248 [606] [607] [608] [609] [610] [611] [612] [613] [614] [615] [616] [617] [618] [619] References Chiesa, F; Sacchini, VS. Risk-reducing mastectomy. Minerva Ginecol, 2016 68(5), 544-547. Newman, LA; Kuerer, HM; Hung, KK; Vlastos, G; Ames, FC; Ross, MI; Singletary, SE. Prophylactic mastectomy. J Am Coll Surg, 2000 191(3), 322-330. Edlich, RF; Winters, KL; Faulkner, BC; Lin, KY. Risk-reducing mastectomy. J Long Term Eff Med Implants, 2006 16(4), 301-314. von Smitten, K. Prophylactic mastectomy: why and when? J Br Menopause Soc, 2003 9(4), 151-155. Tarone, RE; Lipworth, L; Young, VL; McLaughlin, JK. Breast reduction surgery and breast cancer risk: does reduction mammaplasty have a role in primary prevention strategies for women at high risk of breast cancer? Plast Reconstr Surg, 2004 113(7), 2104-2110. Eisinger, F. Prophylactic mastectomy: ethical issues. Br Med Bull, 2007 81-82, 7-19. Basu, NN; Ross, GL; Evans, DG; Barr, L. The Manchester guidelines for contralateral risk-reducing mastectomy. World J Surg Oncol, 2015 13, 237. Eeles, RA. Screening for hereditary cancer and genetic testing, epitomized by breast cancer. Eur J Cancer, 1999 35(14), 1954-1962. Biglia, N; D’Alonzo, M; Sgro, LG; Tomasi Cont, N; Bounous, V; Robba, E. Breast cancer treatment in mutation carriers: surgical treatment. Minerva Ginecol, 2016 68(5), 548-556. Heisey, R; Carroll, JC. Identification and management of women with a family history of breast cancer: Practical guide for clinicians. Can Fam Physician, 2016 62(10), 799-803. Ludwig, KK; Neuner, J; Butler, A; Geurts, JL; Kong, AL. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surg, 2016 212(4), 660-669. Ager, B; Butow, P; Jansen, J; Phillips, KA; Porter, D; CPM DA Advisory Group. Contralateral prophylactic mastectomy (CPM): A systematic review of patient reported factors and psychological predictors influencing choice and satisfaction. Breast, 2016 28, 107-120. Glassey, R; Ives, A; Saunders, C; Musiello, T. Decision making, psychological wellbeing and psychosocial outcomes for high risk women who choose to undergo bilateral prophylactic mastectomy – A review of the literature. Breast, 2016 28, 130-135. Pederson, HJ; Padia, SA; May, M; Grobmyer, S. Managing patients at genetic risk of breast cancer. Cleve Clin J Med, 2016 83(3), 199-206. References [620] [621] [622] [623] [624] [625] [626] [627] [628] [629] [630] [631] [632] [633] 249 Hall, MJ; Obeid, EI; Schwartz, SC; Mantia-Smaldone, G; Forman, AD; Daly, MB. Genetic testing for hereditary cancer predisposition: BRCA1/2, Lynch syndrome, and beyond. Gynecol Oncol, 2016 140(3), 565-574. Peters, ML; Garber, JE; Tung, N. Managing hereditary breast cancer risk in women with and without ovarian cancer. Gynecol Oncol, 2017 146(1), 205-214. Bayraktar, S; Arun, B. BRCA mutation genetic testing implications in the United States. Breast, 2017 31, 224-232. Lejour, M. Reconstructive options after cancer surgery of the breast. Eur J Surg Oncol, 1989 15(6), 496-503. Snyderman, RK. Breast reconstruction today. Breast Cancer Res Treat, 1983 3(1), 5-13. van Smitten, K. Breast reconstruction. Acta Oncol, 1995 34(5), 685-688. Petit, J; Rietjens, M; Garusi, C. Breast reconstructive techniques in cancer patients: which ones, when to apply, which immediate and long term risks? Crit Rev Oncol Hematol, 2001 38(3), 231-239. Dowden, RV; Yetman, RJ. Mastectomy with immediate reconstruction: issues and answers. Cleve Clin J Med, 1992 59(5), 499-503. Ribuffo, D; Monfrecola, A; Guerra, M; Di Benedetto, GM; Grassetti, L; Spaziani, E; Vitagliano, T; Greco, M. Does postoperative radiation therapy represent a contraindication to expander-implant based immediate breast reconstruction? An update 2012-2014. Eur Rev Med Pharmacol Sci, 2015 19(12), 2202-2207. Nestle-Krämling, C; Bölke, E; Budach, W; Andree, C. Breast reconstruction after neoadjuvant radio chemotherapy: review and personal technique IDEAL concept REV-EJMR-D-15-00268. Eur J Med Res, 2016 21(1), 24. Senkus-Konefka, E; Wełnicka-Jaśkiewicz, M; Jaśkiewicz, J; Jassem, J. Radiotherapy for breast cancer in patients undergoing breast reconstruction or augmentation. Cancer Treat Rev, 2004 30(8), 671-682. Shah, C; Kundu, N; Arthur, D; Vicini, F. Radiation therapy following postmastectomy reconstruction: a systematic review. Ann Surg Oncol, 2013 20(4), 1313-1322. El-Sabawi, B; Carey, JN; Hagopian, TM; Sbitany, H; Patel, KM. Radiation and breast reconstruction: Algorithmic approach and evidence-based outcomes. J Surg Oncol, 2016 113(8), 906-912. Oh, E; Chim, H; Soltanian, HT. The effects of neoadjuvant and adjuvant chemotherapy on the surgical outcomes of breast reconstruction. J Plast Reconstr Aesthet Surg, 2012 65(10), e267-e280. 250 [634] [635] [636] [637] [638] [639] [640] [641] [642] [643] [644] [645] [646] [647] [648] [649] References Agrawal, A; Sibbering, DM; Courtney, CA. Skin sparing mastectomy and immediate breast reconstruction: a review. Eur J Surg Oncol, 2013 39(4), 320328. Edlich, RF; Winters, KL; Faulkner, BC; Bill, TJ; Lin, KY. Advances in breast reconstruction after mastectomy. J Long Term Eff Med Implants, 2005 15(2), 197-207. Nava, M; Quattrone, P; Riggio, E. Focus on the breast fascial system: a new approach for inframammary fold reconstruction. Plast Reconstr Surg, 1998 102(4), 1034-1045. van Deventer, PV; Graewe, FR. The Blood Supply of the Breast Revisited. Plast Reconstr Surg, 2016 137(5), 1388-1397. Hang-Fu, L; Snyderman, RK. State-of-the-art breast reconstruction. Cancer, 1991 68(5 Suppl), 1148-1156. Brown, HG. Patient issues in breast reconstruction. Cancer, 1991 68(5 Suppl), 1167-1169. Carlson, GW. Breast reconstruction. Surgical options and patient selection. Cancer, 1994 74(1 Suppl), 436-439. Brinton, LA. Do breast implants after a mastectomy affect subsequent prognosis and survival? Breast Cancer Res, 2005 7(2), 61-63. Lee, KT; Mun, GH. Prosthetic breast reconstruction in previously irradiated breasts: A meta-analysis. J Surg Oncol, 2015 112(5), 468-475. Lee, KT; Mun, GH. Comparison of one-stage vs two-stage prosthesis-based breast reconstruction: a systematic review and meta-analysis. Am J Surg, 2016 212(2), 336-344. Nahabedian, MY. Implant-based breast reconstruction: Strategies to achieve optimal outcomes and minimize complications. J Surg Oncol, 2016 113(8), 895905. Logan Ellis, H; Asaolu, O; Nebo, V; Kasem, A. Biological and synthetic mesh use in breast reconstructive surgery: a literature review. World J Surg Oncol, 2016 14, 121. Spear, SL; Sher, SR; Al-Attar, A. Focus on technique: supporting the soft-tissue envelope in breast reconstruction. Plast Reconstr Surg, 2012 130(5 Suppl 2), 89S-94S. Fisher, J; Hammond, DC. The combination of expanders with autogenous tissue in breast reconstruction. Clin Plast Surg, 1994 21(2), 309-320. Maxwell, GP; Gabriel, A. Bioengineered Breast: Concept, Technique, and Preliminary Results. Plast Reconstr Surg, 2016 137(2), 415-421. Elliott, LF; Hartrampf, CR Jr. Breast reconstruction: progress in the past decade. World J Surg, 1990 14(6), 763-775. References [650] [651] [652] [653] [654] [655] [656] [657] [658] [659] [660] [661] [662] [663] [664] [665] [666] 251 Bostwick, J 3rd. Breast reconstruction after mastectomy. Semin Surg Oncol, 1988 4(4), 274-279. Kroll, SS. Why autologous tissue? Clin Plast Surg, 1998 25(2), 135-143. Hivernaud, V; Lefourn, B; Guicheux, J; Weiss, P; Festy, F; Girard, AC; Roche, R. Autologous Fat Grafting in the Breast: Critical Points and Technique Improvements. Aesthetic Plast Surg, 2015 39(4), 547-561. Nahabedian, MY; Patel, K. Autologous flap breast reconstruction: Surgical algorithm and patient selection. J Surg Oncol, 2016 113(8), 865-874. Coleman, JJ 3rd; Bostwick, J. Rectus abdominis muscle-musculocutaneous flap in chest-wall reconstruction. Surg Clin North Am, 1989 69(5), 1007-1027. Kanchwala, SK; Bucky, LP. Optimizing pedicled transverse rectus abdominis muscle flap breast reconstruction. Cancer J, 2008 14(4), 236-240. Tachi, M; Yamada, A. Choice of flaps for breast reconstruction. Int J Clin Oncol, 2005 10(5), 289-297. Gabert, PE; Bodin, F; Aljudaibi, N; Duquennoy-Martinot, V; Guerreschi, P. The Transverse Musculocutaneous Gracilis Free Flap: Virtual Animation-Assisted Dissection and Application in Breast Reconstruction. Plast Reconstr Surg, 2016 137(5), 1384-1387. Rainsbury, RM. Breast-sparing reconstruction with latissimus dorsi miniflaps. Eur J Surg Oncol, 2002 28(8), 891-895. Hammond, DC. Latissimus dorsi flap breast reconstruction. Clin Plast Surg, 2007 34(1), 75-82. Allen, RJ. The superior gluteal artery perforator flap. Clin Plast Surg, 1998 25(2), 293-302. Shaw, WW. Superior gluteal free flap breast reconstruction. Clin Plast Surg, 1998 25(2), 267-274. Elliott, LF; Hartrampf, CR Jr. The Rubens flap. The deep circumflex iliac artery flap. Clin Plast Surg, 1998 25(2), 283-291. Craigie, JE; Allen, RJ; DellaCroce, FJ; Sullivan, SK. Autogenous breast reconstruction with the deep inferior epigastric perforator flap. Clin Plast Surg, 2003 30(3), 359-369. Galanis, C; Nguyen, P; Koh, J; Roostaeian, J; Festekjian, J; Crisera, C. Microvascular lifeboats: a stepwise approach to intraoperative venous congestion in DIEP flap breast reconstruction. Plast Reconstr Surg, 2014 134(1), 20-27. Allen, RJ; Haddock, NT; Ahn, CY; Sadeghi, A. Breast reconstruction with the profunda artery perforator flap. Plast Reconstr Surg, 2012 129(1), 16e-23e. Mathes DW, Neligan PC. Current techniques in preoperative imaging for abdomen-based perforator flap microsurgical breast reconstruction. J Reconstr 252 [667] [668] [669] [670] [671] [672] [673] [674] [675] [676] [677] [678] [679] References Microsurg. 2010 Jan;26(1):3-10. doi: 10.1055/s-0029-1244806. Epub 2009 Dec 18. Karunanithy, N; Rose, V; Lim, AK; Mitchell, A. CT angiography of inferior epigastric and gluteal perforating arteries before free flap breast reconstruction. Radiographics, 2011 31(5), 1307-1319. Ohkuma, R; Mohan, R; Baltodano, PA; Lacayo, MJ; Broyles, JM; Schneider, EB; Yamazaki, M; Cooney, DS; Manahan, MA; Rosson, GD. Abdominally based free flap planning in breast reconstruction with computed tomographic angiography: systematic review and meta-analysis. Plast Reconstr Surg, 2014 133(3), 483-494. Mathes, DW; Neligan, PC. Preoperative imaging techniques for perforator selection in abdomen-based microsurgical breast reconstruction. Clin Plast Surg, 2010 37(4), 581-591. Avraham, T; Clavin, N; Mehrara, BJ. Microsurgical breast reconstruction. Cancer J, 2008 14(4), 241-247. Patel, NG; Ramakrishnan, V. Microsurgical Tissue Transfer in Breast Reconstruction. Clin Plast Surg, 2017 44(2), 345-359. Shaw, WW; Ahn, CY. Microvascular free flaps in breast reconstruction. Clin Plast Surg, 1992 19(4), 917-926. Ninković, MM; Schwabegger, AH; Anderl, H. Internal mammary vessels as a recipient site. Clin Plast Surg, 1998 25(2), 213-221. Serletti, JM; Moran, SL. Microvascular reconstruction of the breast. Semin Surg Oncol, 2000 19(3), 264-271. Nahabedian, M. The internal mammary artery and vein as recipient vessels for microvascular breast reconstruction. Ann Plast Surg, 2012 68(5), 537-538. Banwell, M; Trotter, D; Ramakrishnan, V. The thoracodorsal artery and vein as recipient vessels for microsurgical breast reconstruction. Ann Plast Surg, 2012 68(5), 542-543. Maher, JL; Mahabir, RC; Roehl, KR. Addressing the Potential Need for Coronary Artery Bypass Grafting After Free Tissue Transfer for Breast Reconstruction: An Algorithmic Approach. Ann Plast Surg, 2015 75(2), 140143. Noszczyk, B. Stem Cell-Assisted Lipotransfer and the Private Breast Surgery Market. Ann Transplant, 2015 20, 526-531. Charvet, HJ; Orbay, H; Wong, MS; Sahar, DE. The Oncologic Safety of Breast Fat Grafting and Contradictions Between Basic Science and Clinical Studies: A Systematic Review of the Recent Literature. Ann Plast Surg, 2015 75(4), 471479. References [680] [681] [682] [683] [684] [685] [686] [687] [688] [689] [690] [691] [692] [693] 253 Bielli, A; Scioli, MG; Gentile, P; Cervelli, V; Orlandi, A. Adipose TissueDerived Stem Cell Therapy for Post-Surgical Breast Reconstruction – More Light than Shadows. Adv Clin Exp Med, 2015 24(3), 545-548. Delay, E; Guerid, S. The Role of Fat Grafting in Breast Reconstruction. Clin Plast Surg, 2015 42(3), 315-323. Petit, JY; Maisonneuve, P; Rotmensz, N; Bertolini, F; Clough, KB; Sarfati, I; Gale, KL; Macmillan, RD; Rey, P; Benyahi, D; Rietjens, M. Safety of Lipofilling in Patients with Breast Cancer. Clin Plast Surg, 2015 42(3), 339-344. Gennari, R; Griguolo, G; Dieci, MV; Guarneri, V; Tavaniello, B; Sibilio, A; Conte, P. Fat grafting for breast cancer patients: From basic science to clinical studies. Eur J Surg Oncol, 2016 42(8), 1088-1102. Waked, K; Colle, J; Doornaert, M; Cocquyt, V; Blondeel, P. Systematic review: The oncological safety of adipose fat transfer after breast cancer surgery. Breast, 2017 31, 128-136. Patrick, CW Jr. Adipose tissue engineering: the future of breast and soft tissue reconstruction following tumor resection. Semin Surg Oncol, 2000 19(3), 302311. Saint-Cyr, M; Rojas, K; Colohan, S; Brown, S. The role of fat grafting in reconstructive and cosmetic breast surgery: a review of the literature. J Reconstr Microsurg, 2012 28(2), 99-110. Krumboeck, A; Giovanoli, P; Plock, JA. Fat grafting and stem cell enhanced fat grafting to the breast under oncological aspects – Recommendations for patient selection. Breast, 2013 22(5), 579-584. Bertolini, F. Contribution of endothelial precursors of adipose tissue to breast cancer: progression-link with fat graft for reconstructive surgery. Ann Endocrinol (Paris), 2013 74(2), 106-107. Philips, BJ; Marra, KG; Rubin, JP. Healing of grafted adipose tissue: current clinical applications of adipose-derived stem cells for breast and face reconstruction. Wound Repair Regen, 2014 22 Suppl 1, 11-13. Kocak, E; Nagel, TW; Hulsen, JH 3rd; Carruthers, KH; Povoski, SP; Salgado, CJ; Chao, AH. Biologic matrices in oncologic breast reconstruction after mastectomy. Expert Rev Med Devices, 2014 11(1), 65-75. Sbitany, H; Langstein, HN. Acellular dermal matrix in primary breast reconstruction. Aesthet Surg J, 2011 31(7 Suppl), 30S-37S. Nahabedian, MY; Spear, SL. Acellular dermal matrix for secondary procedures following prosthetic breast reconstruction. Aesthet Surg J, 2011 31(7 Suppl), 38S-50S. Krishnan, NM; Chatterjee, A; Van Vliet, MM; Powell, SG; Rosen, JM; Nigriny, JF. A comparison of acellular dermal matrix to autologous dermal flaps in 254 [694] [695] [696] [697] [698] [699] [700] [701] [702] [703] [704] [705] [706] References single-stage, implant-based immediate breast reconstruction: a cost-effectiveness analysis. Plast Reconstr Surg, 2013 131(5), 953-961. Potter, S; Browning, D; Savović, J; Holcombe, C; Blazeby, JM. Systematic review and critical appraisal of the impact of acellular dermal matrix use on the outcomes of implant-based breast reconstruction. Br J Surg, 2015 102(9), 10101025. Cheng, A; Saint-Cyr, M. Comparison of different ADM materials in breast surgery. Clin Plast Surg, 2012 39(2), 167-175. Ayeni, OA; Ibrahim, AM; Lin, SJ; Slavin, SA. Acellular dermal matrices in breast surgery: tips and pearls. Clin Plast Surg, 2012 39(2), 177-186. JoAnna Nguyen, T; Carey, JN; Wong, AK. Use of human acellular dermal matrix in implant- based breast reconstruction: evaluating the evidence. J Plast Reconstr Aesthet Surg, 2011 64(12), 1553-1561. Nahabedian, MY. Nipple reconstruction. Clin Plast Surg, 2007 34(1), 131-137. Momeni, A; Becker, A; Torio-Padron, N; Iblher, N; Stark, GB; Bannasch, H. Nipple reconstruction: evidence-based trials in the plastic surgical literature. Aesthetic Plast Surg, 2008 32(1), 18-20. Farhadi, J; Maksvytyte, GK; Schaefer, DJ; Pierer, G; Scheufler, O. Reconstruction of the nipple-areola complex: an update. J Plast Reconstr Aesthet Surg, 2006 59(1), 40-53. Winocour, S; Saksena, A; Oh, C; Wu, PS; Laungani, A; Baltzer, H; Saint-Cyr, M. A Systematic Review of Comparison of Autologous, Allogeneic, and Synthetic Augmentation Grafts in Nipple Reconstruction. Plast Reconstr Surg, 2016 137(1), 14e-23e. Sisti, A; Grimaldi, L; Tassinari, J; Cuomo, R; Fortezza, L; Bocchiotti, MA; Roviello, F; D’Aniello, C; Nisi, G. Nipple-areola complex reconstruction techniques: A literature review. Eur J Surg Oncol, 2016 42(4), 441-465. Boccola, MA; Savage, J; Rozen, WM; Ashton, MW; Milner, C; Rahdon, R; Whitaker, IS. Surgical correction and reconstruction of the nipple-areola complex: current review of techniques. J Reconstr Microsurg, 2010 26(9), 589600. Alipour, S; Eskandari, A. Systematic review of effects of pregnancy on breast and abdominal contour after TRAM/DIEP breast reconstruction in breast cancer survivors. Breast Cancer Res Treat, 2015 152(1), 9-15. Collin, TW; Coady, MS. Is pregnancy contraindicated following free TRAM breast reconstruction? J Plast Reconstr Aesthet Surg, 2006 59(5), 556-559. Erić, M; Mihić, N; Krivokuća, D. Breast reconstruction following mastectomy; patient’s satisfaction. Acta Chir Belg, 2009 109(2), 159-166. References [707] [708] [709] [710] [711] [712] [713] [714] [715] [716] [717] [718] [719] [720] 255 Chan, LK. Body image and the breast: the psychological wound. J Wound Care, 2010 19(4), 133-134, 136, 138. Pusic, AL; Klassen, AF; Snell, L; Cano, SJ; McCarthy, C; Scott, A; Cemal, Y; Rubin, LR; Cordeiro, PG. Measuring and managing patient expectations for breast reconstruction: impact on quality of life and patient satisfaction. Expert Rev Pharmacoecon Outcomes Res, 2012 12(2), 149-158. Reavey, P; McCarthy, CM. Update on breast reconstruction in breast cancer. Curr Opin Obstet Gynecol, 2008 20(1), 61-67. Maass, SW; Bagher, S; Hofer, SO; Baxter, NN; Zhong, T. Systematic Review: Aesthetic Assessment of Breast Reconstruction Outcomes by Healthcare Professionals. Ann Surg Oncol, 2015 22(13), 4305-4316. Korus, LJ; Cypel, T; Zhong, T; Wu, AW. Patient-reported outcome measures in reconstructive breast surgery: is there a role for generic measures? Plast Reconstr Surg, 2015 135(3), 479e-490e. Guyomard, V; Leinster, S; Wilkinson, M. Systematic review of studies of patients’ satisfaction with breast reconstruction after mastectomy. Breast, 2007 16(6), 547-567. Shridharani, SM; Magarakis, M; Stapleton, SM; Basdag, B; Seal, SM; Rosson, GD. Breast sensation after breast reconstruction: a systematic review. J Reconstr Microsurg, 2010 26(5), 303-310. Fingeret, MC; Nipomnick, SW; Crosby, MA; Reece, GP. Developing a theoretical framework to illustrate associations among patient satisfaction, body image and quality of life for women undergoing breast reconstruction. Cancer Treat Rev, 2013 39(6), 673-681. Cohen, WA; Mundy, LR; Ballard, TN; Klassen, A; Cano, SJ; Browne, J; Pusic, AL. The BREAST-Q in surgical research: A review of the literature 2009-2015. J Plast Reconstr Aesthet Surg, 2016 69(2), 149-162. Cano, SJ; Klassen, AF; Scott, AM; Pusic, AL. A closer look at the BREASTQ(©). Clin Plast Surg, 2013 40(2), 287-296. Wood, WC. Nonimaging aspects of follow-up in breast cancer reconstruction. Cancer, 1991 68(5 Suppl), 1164-1166. Zakhireh, J; Fowble, B; Esserman, LJ. Application of screening principles to the reconstructed breast. J Clin Oncol, 2010 28(1), 173-180. Scaranelo, AM; Lord, B; Eiada, R; Hofer, SO. Imaging approaches and findings in the reconstructed breast: a pictorial essay. Can Assoc Radiol J, 2011 62(1), 60-72. Adrada, BE; Whitman, GJ; Crosby, MA; Carkaci, S; Dryden, MJ; Dogan, BE. Multimodality Imaging of the Reconstructed Breast. Curr Probl Diagn Radiol, 2015 44(6), 487-495. 256 [721] [722] [723] [724] [725] [726] [727] [728] [729] [730] [731] [732] [733] [734] [735] [736] [737] [738] References Salzberg, CA. Barbed sutures in breast reconstruction. Aesthet Surg J, 2013 33(3 Suppl), 40S-43S. Mitchell, RT; Bengtson, BP. Clinical Applications of Barbed Suture in Aesthetic Breast Surgery. Clin Plast Surg, 2015 42(4), 595-604. Kincaid, SB. Breast reconstruction: a review. Ann Plast Surg, 1984 12(5), 431448. Kroll, SS. Bilateral breast reconstruction. Clin Plast Surg, 1998 25(2), 251-259. Bostwick, J 3rd. Reconstruction after mastectomy. Surg Clin North Am, 1990 70(5), 1125-1140. Daniel, RK; Maxwell, GP. Breast reconstruction following mastectomy. Adv Surg, 1983 16, 49-73. Rizki, H; Nkonde, C; Ching, RC; Kumiponjera, D; Malata, CM. Plastic surgical management of the contralateral breast in post-mastectomy breast reconstruction. Int J Surg, 2013 11(9), 767-772. Dinner, MI. Postmastectomy reconstruction. Surg Clin North Am, 1984 64(6), 1193-1207. Gainer, SM; Lucci, A. Oncoplastics: techniques for reconstruction of partial breast defects based on tumor location. J Surg Oncol, 2011 103(4), 341-347. Dobke, M. Impact of advances in breast cancer management on reconstructive and aesthetic breast surgery. Clin Plast Surg, 2012 39(4), 465-475. Tansley, P; Ramsey, K; Wong, S; Guerrieri, M; Pitcher, M; Grinsell, D. New treatment sequence protocol to reconstruct locally advanced breast cancer. ANZ J Surg, 2013 83(9), 630-635. DellaCroce, FJ; Wolfe, ET. Breast reconstruction. Surg Clin North Am, 2013 93(2), 445-454. Ballard, TN; Momoh, AO. Advances in breast reconstruction of mastectomy and lumpectomy defects. Surg Oncol Clin N Am, 2014 23(3), 525-548. O’Connell, RL; Stevens, RJ; Harris, PA; Rusby, JE. Review of threedimensional (3D) surface imaging for oncoplastic, reconstructive and aesthetic breast surgery. Breast, 2015 24(4), 331-342. Momeni, A; Kovach, SJ. Important considerations in chest wall reconstruction. J Surg Oncol, 2016 113(8), 913-922. Savalia, NB; Silverstein, MJ. Oncoplastic breast reconstruction: Patient selection and surgical techniques. J Surg Oncol, 2016 113(8), 875-882. Farhangkhoee, H; Matros, E; Disa, J. Trends and concepts in post-mastectomy breast reconstruction. J Surg Oncol, 2016 113(8), 891-894. Srinivasaiah, N; Drew, PJ; Platt, A. Quality of life issues in aesthetic breast surgery. Br J Hosp Med (Lond), 2010 71(4), 211-215. References [739] [740] [741] [742] [743] [744] [745] [746] [747] [748] [749] [750] [751] [752] [753] [754] 257 Elsahy, N. Recent advances in the treatment of hypertrophy and ptosis of the breast. J Med Assoc Ga, 1991 80(11), 627-630. Xi, W; Perdanasari, AT; Ong, Y; Han, S; Min, P; Su, W; Feng, S; Pacchioni, L; Zhang, YX; Lazzeri, D. Objective breast volume, shape and surface area assessment: a systematic review of breast measurement methods. Aesthetic Plast Surg, 2014 38(6), 1116-1130. Hall-Findlay, EJ. The three breast dimensions: analysis and effecting change. Plast Reconstr Surg, 2010 125(6), 1632-1642. Yang, J; Zhang, R; Shen, J; Hu, Y; Lv, Q. The Three-Dimensional Techniques in the Objective Measurement of Breast Aesthetics. Aesthetic Plast Surg, 2015 39(6), 910-915. Blondeel, PN; Hijjawi, J; Depypere, H; Roche, N; Van Landuyt, K. Shaping the breast in aesthetic and reconstructive breast surgery: an easy three-step principle. Plast Reconstr Surg, 2009 123(2), 455-462. Mallucci, P; Branford, OA. Shapes, Proportions, and Variations in Breast Aesthetic Ideals: The Definition of Breast Beauty, Analysis, and Surgical Practice. Clin Plast Surg, 2015 42(4), 451-464. Gefen, A; Dilmoney, B. Mechanics of the normal woman’s breast. Technol Health Care, 2007 15(4), 259-271. Gutowski, KA. Aesthetic and functional breast surgery. Clin Obstet Gynecol, 2006 49(2), 337-345. Nguyen, JT; Wheatley, MJ; Schnur, PL; Nguyen, TA; Winn, SR. Reduction mammaplasty: a review of managed care medical policy coverage criteria. Plast Reconstr Surg, 2008 121(4), 1092-1100. Field, DA; Miller, S. Cosmetic breast surgery. Am Fam Physician, 1992 45(2), 711-719. Ergün, SS; Gayretli, Ö; Kayan, RB. Brassiere strap groove deformity: definition and classification. Aesthetic Plast Surg, 2014 38(2), 350-353. Howrigan, PJ. Reduction and augmentation mammoplasty. Obstet Gynecol Clin North Am, 1994 21(3), 539-549. Pusic, AL; McCarthy, C; Cano, SJ; Klassen, AF; Kerrigan, CL. Clinical research in breast surgery: reduction and postmastectomy reconstruction. Clin Plast Surg, 2008 35(2), 215-226. Nahabedian, MY. Breast deformities and mastopexy. Plast Reconstr Surg, 2011 127(4), 91e-102e. Krupp, S. Mastopexy: modification of periwinkle shell operation. Ten years of experience. Aesthetic Plast Surg, 1990 14(1), 9-14. Hidalgo, DA; Spector, JA. Mastopexy. Plast Reconstr Surg, 2013 132(4), 642e656e. 258 [755] [756] [757] [758] [759] [760] [761] [762] [763] [764] [765] [766] [767] [768] [769] References Rohrich, RJ; Thornton, JF; Jakubietz, RG; Jakubietz, MG; Grünert, JG. The limited scar mastopexy: current concepts and approaches to correct breast ptosis. Plast Reconstr Surg, 2004 114(6), 1622-1630. Raposo-Amaral, CE; Raposo-Amaral, CM; Marques, FF; Denadai, R; RaposoAmaral, CA. The inverted-T mammaplasty: a modified winch suture to reduce horizontal scar length. Aesthet Surg J, 2014 34(1), 183-188. Hall-Findlay, EJ; Shestak, KC. Breast Reduction. Plast Reconstr Surg, 2015 136(4), 531e-544e. Andrades, P; Prado, A. Understanding modern breast reduction techniques with a simplified approach. J Plast Reconstr Aesthet Surg, 2008 61(11), 1284-1293. Kalliainen, LK; ASPS Health Policy Committee. ASPS clinical practice guideline summary on reduction mammaplasty. Plast Reconstr Surg, 2012 130(4), 785-789. McGregor, JC. The changing scene in breast surgery in a plastic surgery unit (1966-94). J R Coll Surg Edinb, 1995 40(5), 279-289. Jones, SA; Bain, JR. Review of data describing outcomes that are used to assess changes in quality of life after reduction mammaplasty. Plast Reconstr Surg, 2001 108(1), 62-67. Chiari, A Jr. The L short-scar mammaplasty. Clin Plast Surg, 2002 29(3), 401409. Hall-Findlay, EJ. Pedicles in vertical breast reduction and mastopexy. Clin Plast Surg, 2002 29(3), 379-391. Regnault, P. Breast reduction and mastopexy, an old love story: B technique update. Aesthetic Plast Surg, 1990 14(2), 101-106. Meyer, R. The principles of the up-to-date breast reduction. Aesthetic Plast Surg, 1990 14(1), 1-7. Gargano, F; Tessier, P; Wolfe, SA. Breast reduction with dermoglandular flaps: Tessier’s “total dermo-mastopexy” and the “yin-yang technique.” Ann Plast Surg, 2011 67(6), S42-S54. O’Dey, DM; Demir, E; Pallua, N. The bivectorial full-thickness superiorly based NAC flap: a new option to increase plasticity and decrease tension in the superior pedicle vertical mammaplasty technique. Aesthetic Plast Surg, 2008 32(5), 802-804. Hudson, DA; Geldenhuys, S; Duminy, F; Adams, K. Another look at breast projection after breast reduction. Aesthetic Plast Surg, 2008 32(6), 928-932. Atiyeh, BS; Rubeiz, MT; Hayek, SN. Refinements of vertical scar mammaplasty: circumvertical skin excision design with limited inferior pole subdermal undermining and liposculpture of the inframammary crease. Aesthetic Plast Surg, 2005 29(6), 519-531. References [770] [771] [772] [773] [774] [775] [776] [777] [778] [779] [780] [781] [782] [783] [784] [785] 259 Mottura, AA. Circumvertical reduction mammaplasty. Clin Plast Surg, 2002 29(3), 393-399. Poëll, JG. Vertical reduction mammaplasty. Aesthetic Plast Surg, 2004 28(2), 59-69. Chen, CM; Warren, SM; Isik, FF. Innovations to the vertical reduction mammaplasty: making the transition. Ann Plast Surg, 2003 50(6), 579-587. Lalonde, DH; Lalonde, J; French, R. The no vertical scar breast reduction: a minor variation that allows to remove vertical scar portion of the inferior pedicle wise pattern T scar. Aesthetic Plast Surg, 2003 27(5), 335-344. Hammond, DC. The SPAIR mammaplasty. Clin Plast Surg, 2002 29(3), 411421. Matarasso, A. Suction mammaplasty: the use of suction lipectomy alone to reduce large breasts. Clin Plast Surg, 2002 29(3), 433-443. Jakubietz, RG; Jakubietz, DF; Gruenert, JG; Schmidt, K; Meffert, RH; Jakubietz, MG. Breast reduction by liposuction in females. Aesthetic Plast Surg, 2011 35(3), 402-407. McCulley, SJ; Macmillan, RD. Planning and use of therapeutic mammoplasty – Nottingham approach. Br J Plast Surg, 2005 58(7), 889-901. Macmillan, RD; James, R; Gale, KL; McCulley, SJ. Therapeutic mammaplasty. J Surg Oncol, 2014 110(1), 90-95. Hernanz, F; Regaño, S; Vega, A; Gómez Fleitas, M. Reduction mammaplasty: an advantageous option for breast conserving surgery in large-breasted patients. Surg Oncol, 2010 19(4), e95-e102. Thibaudeau, S; Sinno, H; Williams, B. The effects of breast reduction on successful breastfeeding: a systematic review. J Plast Reconstr Aesthet Surg, 2010 63(10), 1688-1693. Shestak, KC; Davidson, EH. Assessing Risk and Avoiding Complications in Breast Reduction. Clin Plast Surg, 2016 43(2), 323-331. Ortiz-Pomales, YT; Priyanka, H; Newell, MS; Losken, A. Reduction Mammaplasty and Breast Cancer Screening. Clin Plast Surg, 2016 43(2), 333339. Carlson, GW. The Management of Breast Cancer Detected by Reduction Mammaplasty. Clin Plast Surg, 2016 43(2), 341-347. Hansen, JE. Avoiding the Unfavorable Outcome with Wise Pattern Breast Reduction. Clin Plast Surg, 2016 43(2), 349-358. Misani, M; De Mey, A. Managing Complications in Vertical Mammaplasty. Clin Plast Surg, 2016 43(2), 359-363. 260 [786] [787] [788] [789] [790] [791] [792] [793] [794] [795] [796] [797] [798] [799] [800] [801] [802] References Hammond, DC; Kim, K. The Short Scar Periareolar Inferior Pedicle Reduction Mammaplasty: Management of Complications. Clin Plast Surg, 2016 43(2), 365372. Austin, RE; Lista, F; Ahmad, J. Management of Recurrent or Persistent Macromastia. Clin Plast Surg, 2016 43(2), 383-393. Garcia, O Jr. Management of Asymmetry after Breast Reduction. Clin Plast Surg, 2016 43(2), 373-382. Spear, SL; Albino, FP. Management of the High-Riding Nipple after Breast Reduction. Clin Plast Surg, 2016 43(2), 395-401. Rancati, A; Irigo, M; Angrigiani, C. Management of the Ischemic Nipple-Areola Complex after Breast Reduction. Clin Plast Surg, 2016 43(2), 403-414. Handel, N; Yegiyants, S. Managing Necrosis of the Nipple Areolar Complex Following Reduction Mammaplasty and Mastopexy. Clin Plast Surg, 2016 43(2), 415-423. Powers, KL; Phillips, LG. Breast Reduction in the Burned Breast. Clin Plast Surg, 2016 43(2), 425-428. Kling, RE; Tobler, WD Jr; Gusenoff, JA; Rubin, JP. Avoiding Complications in Gigantomastia. Clin Plast Surg, 2016 43(2), 429-439. Reisman, NR. Medicolegal Issues in Breast Reduction. Clin Plast Surg, 2016 43(2), 441-444. Bengtson, BP; Baxter, RA. Emerging applications for acellular dermal matrices in mastopexy. Clin Plast Surg, 2012 39(2), 159-166. Miotto, GC; Eaves, FF 3rd. The Circumrotational Technique for Mastopexy. Aesthet Surg J, 2015 35(7), 796-809. Schleich, AR; Black, DM; McCraw, JB. The aesthetic correction of the ptotic breast by the procedure of nipple-areola transposition – A contemporary translation and commentary. J Plast Reconstr Aesthet Surg, 2010 63(7), 11361141. Maxwell, GP; Gabriel, A. The evolution of breast implants. Clin Plast Surg, 2009 36(1), 1-13. Adams, WP Jr; Mallucci, P. Breast augmentation. Plast Reconstr Surg, 2012 130(4), 597e-611e. Hidalgo, DA; Spector, JA. Breast augmentation. Plast Reconstr Surg, 2014 133(4), 567e-583e. Elliott, LF. Circumareolar mastopexy with augmentation. Clin Plast Surg, 2002 29(3), 337-347. Sampaio Góes, JC. Periareolar mammaplasty: double-skin technique with application of mesh support. Clin Plast Surg, 2002 29(3), 349-364. References [803] [804] [805] [806] [807] [808] [809] [810] [811] [812] [813] [814] [815] [816] [817] 261 Dunn, KW; Hall, PN; Khoo, CT. Breast implant materials: sense and safety. Br J Plast Surg, 1992 45(4), 315-321. Cohney, BC; Cohney, TB; Hearne, VA. Augmentation mammaplasty – A further review of 20 years using the polyurethane-covered prosthesis. J Long Term Eff Med Implants, 1992 1(3), 269-279. Frame, J; Kamel, D; Olivan, M; Cintra, H. The In Vivo Pericapsular Tissue Response to Modern Polyurethane Breast Implants. Aesthetic Plast Surg, 2015 39(5), 713-723. Scarpa, C; Borso, GF; Vindigni, V; Bassetto, F. Polyurethane foam-covered breast implants: a justified choice? Eur Rev Med Pharmacol Sci, 2015 19(9), 1600-1606. Henderson, PW; Nash, D; Laskowski, M; Grant, RT. Objective Comparison of Commercially Available Breast Implant Devices. Aesthetic Plast Surg, 2015 39(5), 724-732. Spear, SL; Jespersen, MR. Breast implants: saline or silicone? Aesthet Surg J, 2010 30(4), 557-570. Spear, SL; Hedén, P. Allergan’s silicone gel breast implants. Expert Rev Med Devices, 2007 4(5), 699-708. Hedén, P; Montemurro, P; Adams, WP Jr; Germann, G; Scheflan, M; Maxwell, GP. Anatomical and Round Breast Implants: How to Select and Indications for Use. Plast Reconstr Surg, 2015 136(2), 263-272. Schwartz, MR. Algorithm and techniques for using Sientra’s silicone gel shaped implants in primary and revision breast augmentation. Plast Reconstr Surg, 2014 134(1 Suppl), 18S-27S. Calobrace, MB. The design and engineering of the MemoryShape breast implant. Plast Reconstr Surg, 2014 134(3 Suppl), 10S-15S. McCleave, MJ. Is breast augmentation using hyaluronic acid safe? Aesthetic Plast Surg, 2010 34(1), 65-68. Siebert, T; Chaput, B; Vaysse, C; Meresse, T; Chavoin, JP; Garrido, I; Grolleau, JL. The latest information on Macrolane™: its indications and restrictions. Ann Chir Plast Esthet, 2014 59(2), e1-e11. Molitor, M; Měšťák, O; Kalinová, L; Krajcová, A; Měšťák, J. The history and safety of breast implants. Acta Chir Plast, 2014 56(1-2), 15-19. Van Zele, D; Heymans, O. Breast implants. A review. Acta Chir Belg, 2004 104(2), 158-165. Cruz, NI. Current status of silicone breast implants. Bol Asoc Med P R, 1991 83(8), 326-328. 262 [818] [819] [820] [821] [822] [823] [824] [825] [826] [827] [828] [829] [830] [831] [832] [833] [834] References Derby, BM; Codner, MA. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg, 2015 135(1), 113-124. Chao, AH; Garza, R 3rd; Povoski, SP. A review of the use of silicone implants in breast surgery. Expert Rev Med Devices, 2016 13(2), 143-156. Sarwer, DB; Nordmann, JE; Herbert, JD. Cosmetic breast augmentation surgery: a critical overview. J Womens Health Gend Based Med, 2000 9(8), 843-856. Allen, M; Oberle, K. Augmentation mammoplasty: a complex choice. Health Care Women Int, 1996 17(1), 81-90. Fisher, JC; Brody, GS. Breast implants under siege: an historical commentary. J Long Term Eff Med Implants, 1992 1(3), 243-253. Zones, JS. The political and social context of silicone breast implant use in the United States. J Long Term Eff Med Implants, 1992 1(3), 225-241. Friedman, RJ. Silicone breast prostheses implantation and explanation. Semin Arthritis Rheum, 1994 24(1 Suppl 1), 8-10. Gampper, TJ; Khoury, H; Gottlieb, W; Morgan, RF. Silicone gel implants in breast augmentation and reconstruction. Ann Plast Surg, 2007 59(5), 581-590. Yoshida, SH; Chang, CC; Teuber, SS; Gershwin, ME. Silicon and silicone: theoretical and clinical implications of breast implants. Regul Toxicol Pharmacol, 1993 17(1), 3-18. Kossovsky, N; Freiman, CJ. Immunology of silicone breast implants. J Biomater Appl, 1994 8(3), 237-246. Azavedo, E; Boné, B. Imaging breasts with silicone implants. Eur Radiol, 1999 9(2), 349-355. Glynn, C; Litherland, J. Imaging breast augmentation and reconstruction. Br J Radiol, 2008 81(967), 587-595. Steinbach, BG; Hardt, NS; Abbitt, PL. Mammography: breast implants – Types, complications, and adjacent breast pathology. Curr Probl Diagn Radiol, 1993 22(2), 39-86. Uematsu, T. Screening and diagnosis of breast cancer in augmented women. Breast Cancer, 2008 15(2), 159-164. Gorczyca, DP. MR imaging of breast implants. Magn Reson Imaging Clin N Am, 1994 2(4), 659-672. Lui, CY; Ho, CM; Iu, PP; Cheung, WY; Lam, HS; Cheng, MS; Liu, HL. Evaluation of MRI findings after polyacrylamide gel injection for breast augmentation. AJR Am J Roentgenol, 2008 191(3), 677-688. Shah, M; Tanna, N; Margolies, L. Magnetic resonance imaging of breast implants. Top Magn Reson Imaging, 2014 23(6), 345-353. References [835] [836] [837] [838] [839] [840] [841] [842] [843] [844] [845] [846] [847] [848] [849] 263 Creasman, CN; Mordaunt, D; Liolios, T; Chiu, C; Gabriel, A; Maxwell, GP. Four-dimensional breast imaging, part I: introduction of a technology-driven, evidence-based approach to breast augmentation planning. Aesthet Surg J, 2011 31(8), 914-924. Epstein, MD; Scheflan, M. Three-dimensional Imaging and Simulation in Breast Augmentation: What Is the Current State of the Art? Clin Plast Surg, 2015 42(4), 437-450. Venkataraman, S; Hines, N; Slanetz, PJ. Challenges in mammography: part 2, multimodality review of breast augmentation – Imaging findings and complications. AJR Am J Roentgenol, 2011 197(6), W1031-W1045. McIntosh, SA; Horgan, K. Augmentation mammoplasty: effect on diagnosis of breast cancer. J Plast Reconstr Aesthet Surg, 2008 61(2), 124-129. Michalopoulos, K. The effects of breast augmentation surgery on future ability to lactate. Breast J, 2007 13(1), 62-67. Widdice, L. The effects of breast reduction and breast augmentation surgery on lactation: an annotated bibliography. J Hum Lact, 1993 9(3), 161-167. Soderstrom, B. Helping the woman who has had breast surgery: a literature review. J Hum Lact, 1993 9(3), 169-171. Yang, N; Muradali, D. The augmented breast: a pictorial review of the abnormal and unusual. AJR Am J Roentgenol, 2011 196(4), W451-W460. Sinno, S; Wilson, S; Brownstone, N; Levine, SM. Current Thoughts on Fat Grafting: Using the Evidence to Determine Fact or Fiction. Plast Reconstr Surg, 2016 137(3), 818-824. Rosing, JH; Wong, G; Wong, MS; Sahar, D; Stevenson, TR; Pu, LL. Autologous fat grafting for primary breast augmentation: a systematic review. Aesthetic Plast Surg, 2011 35(5), 882-890. Mizuno, H; Hyakusoku, H. Fat grafting to the breast and adipose-derived stem cells: recent scientific consensus and controversy. Aesthet Surg J, 2010 30(3), 381-387. Parrish, JN; Metzinger, SE. Autogenous fat grafting and breast augmentation: a review of the literature. Aesthet Surg J, 2010 30(4), 549-556. Al Sufyani, MA; Al Hargan, AH; Al Shammari, NA; Al Sufyani, MA. Autologous Fat Transfer for Breast Augmentation: A Review. Dermatol Surg, 2016 42(11), 1235-1242. ELFadl, D; Garimella, V; Mahapatra, TK; McManus, PL; Drew, PJ. Lipomodelling of the breast: a review. Breast, 2010 19(3), 202-209. Leopardi, D; Thavaneswaran, P; Mutimer, KL; Olbourne, NA; Maddern, GJ. Autologous fat transfer for breast augmentation: a systematic review. ANZ J Surg, 2014 84(4), 225-230. 264 [850] [851] [852] [853] [854] [855] [856] [857] [858] [859] [860] [861] [862] [863] [864] [865] [866] References Sampaio Goes, JC; Munhoz, AM; Gemperli, R. The Subfascial Approach to Primary and Secondary Breast Augmentation with Autologous Fat Grafting and Form-Stable Implants. Clin Plast Surg, 2015 42(4), 551-564. Coleman, SR; Saboeiro, AP. Primary Breast Augmentation with Fat Grafting. Clin Plast Surg, 2015 42(3), 301-306. Auclair, E; Anavekar, N. Combined Use of Implant and Fat Grafting for Breast Augmentation. Clin Plast Surg, 2015 42(3), 307-314. Maxwell, GP; Gabriel, A. Acellular dermal matrix for reoperative breast augmentation. Plast Reconstr Surg, 2014 134(5), 932-938. Kaufman, D. Pocket reinforcement using acellular dermal matrices in revisionary breast augmentation. Clin Plast Surg, 2012 39(2), 137-148. Hwan, KY; Eup, HS. Useful application of negative suction drainage on the umbilicus after transaxillary breast augmentation. Aesthetic Plast Surg, 2012 36(4), 1002-1004. Munhoz, AM; Gemperli, R; Sampaio Goes, JC. Transaxillary Subfascial Augmentation Mammaplasty with Anatomic Form-Stable Silicone Implants. Clin Plast Surg, 2015 42(4), 565-584. Strock, LL. Surgical Approaches to Breast Augmentation: The Transaxillary Approach. Clin Plast Surg, 2015 42(4), 585-593. Brown, MH; Somogyi, RB; Aggarwal, S. Secondary Breast Augmentation. Plast Reconstr Surg, 2016 138(1), 119e-135e. Spring, MA; Macias, LH; Nadeau, M; Stevens, WG. Secondary augmentationmastopexy: indications, preferred practices, and the treatment of complications. Aesthet Surg J, 2014 34(7), 1018-1040. Matarasso, A; Smith, DM. Combined breast surgery and abdominoplasty: strategies for success. Plast Reconstr Surg, 2015 135(5), 849e-860e. Kroll, SS; Singletary, SE. Repair of partial mastectomy defects. Clin Plast Surg, 1998 25(2), 303-310. MacLennan, SE; Wells, MD; Neale, HW. Reconstruction of the burned breast. Clin Plast Surg, 2000 27(1), 113-119. McGrath, MH. The psychological safety of breast implant surgery. Plast Reconstr Surg, 2007 120(7 Suppl 1), 103S-109S. Sarwer DB. The psychological aspects of cosmetic breast augmentation. Plast Reconstr Surg. 2007 Dec;120(7 Suppl 1):110S-117S. Jacobson, N. The socially constructed breast: breast implants and the medical construction of need. Am J Public Health, 1998 88(8), 1254-1261. Given, KS; Stowers, RG. Breast augmentation: a current controversy. J Med Assoc Ga, 1991 80(11), 617-620. References [867] [868] [869] [870] [871] [872] [873] [874] [875] [876] [877] [878] [879] [880] [881] [882] [883] 265 Atwood, HD; Goodman, RC; Pope, NA; Stuckey, JG; Bates, R; Lehmberg, RW; Pullman, NK; Talbert, GE; Beckman, JS; McCutcheon, FB; et al. The silicone gel breast implant controversy: current status and clinical implications. J Ark Med Soc, 1994 90(9), 427-434. McGrath, MH; Burkhardt, BR. The safety and efficacy of breast implants for augmentation mammaplasty. Plast Reconstr Surg, 1984 74(4), 550-560. Antoniuk, PM. Breast augmentation and breast reduction. Obstet Gynecol Clin North Am, 2002 29(1), 103-115. Backovic, A; Wolfram, D. Silicone mammary implants – Can we turn back the time? Exp Gerontol, 2007 42(8), 713-718. Maxwell, GP; Gabriel, A. The evolution of breast implants. Plast Reconstr Surg, 2014 134(1 Suppl), 12S-17S. Goldberg, P; Habal, MB. Future directions in breast implant surgery. Clin Plast Surg, 2001 28(4), 687-702. Cook, RR; Harrison, MC; LeVier, RR. The breast implant controversy. Arthritis Rheum, 1994 37(2), 153-157. Chopra, K; Gowda, AU; Kwon, E; Eagan, M; Grant Stevens, W. Techniques to Repair Implant Malposition after Breast Augmentation: A Review. Aesthet Surg J, 2016 36(6), 660-671. Lim, GH; Tan, HF. Surgical techniques to avoid lateral dog ear of the mastectomy scar: A systematic review. Int J Surg, 2016 26, 73-78. Vitug, AF; Newman, LA. Complications in breast surgery. Surg Clin North Am, 2007 87(2), 431-451. Frassica, DA; Bajaj, GK; Tsangaris, TN. Treatment of complications after breast-conservation therapy. Oncology (Williston Park), 2003 17(8), 1118-1128. Stubblefield, MD; Custodio, CM. Upper-extremity pain disorders in breast cancer. Arch Phys Med Rehabil, 2006 87(3 Suppl 1), S96-S99. Loughran, CF; Keeling, CR. Seeding of tumour cells following breast biopsy: a literature review. Br J Radiol, 2011 84(1006), 869-874. Robertson, EG; Baxter, G. Tumour seeding following percutaneous needle biopsy: the real story! Clin Radiol, 2011 66(11), 1007-1014. Bates, T; Davidson, T; Mansel, RE. Litigation for pneumothorax as a complication of fine-needle aspiration of the breast. Br J Surg, 2002 89(2), 134137. Piro, AJ; Hellman, S. Effect of primary treatment modality on the metastatic pattern of mammary carcinoma. Cancer Treat Rep, 1978 62(9), 1275-1280. Howell, A. An early peak of relapse after surgery for breast cancer. Breast Cancer Res, 2004 6(6), 255-257. 266 [884] [885] [886] [887] [888] [889] [890] [891] [892] [893] [894] [895] [896] [897] [898] References Baum, M. Does the act of surgery provoke activation of “latent” metastases in early breast cancer? Breast Cancer Res, 2004 6(4), 160-161. Neves-E-Castro, M. Why do some breast cancer cells remain dormant? Gynecol Endocrinol, 2006 22(4), 190-197. Goldfarb, Y; Ben-Eliyahu, S. Surgery as a risk factor for breast cancer recurrence and metastasis: mediating mechanisms and clinical prophylactic approaches. Breast Dis, 2006-2007 26, 99-114. Retsky, MW; Demicheli, R; Hrushesky, WJ; Baum, M; Gukas, ID. Dormancy and surgery-driven escape from dormancy help explain some clinical features of breast cancer. APMIS, 2008 116(7-8), 730-741. Park, Y; Kitahara, T; Takagi, R; Kato, R. Does surgery for breast cancer induce angiogenesis and thus promote metastasis? Oncology, 2011 81(3-4), 199-205. Demicheli, R. Tumour dormancy: findings and hypotheses from clinical research on breast cancer. Semin Cancer Biol, 2001 11(4), 297-306. Ames, FC; Balch, CM. Management of local and regional recurrence after mastectomy or breast-conserving treatment. Surg Clin North Am, 1990 70(5), 1115-1124. Halyard, MY; Wasif, N; Harris, EE; Arthur, DW; Bailey, L; Bellon, JR; Carey, L; Goyal, S; Horst, KC; Moran, MS; MacDonald, SM; Haffty, BG; Expert Panel on Radiation Oncology – Breast. ACR Appropriateness Criteria® local-regional recurrence (LR) and salvage surgery: breast cancer. Am J Clin Oncol, 2012 35(2), 178-182. Osborne, MP. Salvage mastectomy. Semin Surg Oncol, 1991 7(5), 291-295. van der Ploeg, IM; Nieweg, OE; van Rijk, MC; Valdés Olmos, RA; Kroon, BB. Axillary recurrence after a tumour-negative sentinel node biopsy in breast cancer patients: A systematic review and meta-analysis of the literature. Eur J Surg Oncol, 2008 34(12), 1277-1284. Lebya, K; Garcia-Smith, R; Swaminathan, R; Jones, A; Russell, J; Joste, N; Bisoffi, M; Trujillo, K. Towards a personalized surgical margin for breast conserving surgery – Implications of field cancerization in local recurrence. J Surg Oncol, 2017 115(2), 109-115. Baddour, LM. Breast cellulitis complicating breast conservation therapy. J Intern Med, 1999 245(1), 5-9. Simon, MS; Cody, RL. Cellulitis after axillary lymph node dissection for carcinoma of the breast. Am J Med, 1992 93(5), 543-548. Pogson, CJ; Adwani, A; Ebbs, SR. Seroma following breast cancer surgery. Eur J Surg Oncol, 2003 29(9), 711-717. Soo, MS; Williford, ME. Seromas in the breast: imaging findings. Crit Rev Diagn Imaging, 1995 36(5), 385-440. References [899] [900] [901] [902] [903] [904] [905] [906] [907] [908] [909] [910] [911] [912] [913] 267 Turner, EJ; Benson, JR; Winters, ZE. Techniques in the prevention and management of seromas after breast surgery. Future Oncol, 2014 10(6), 10491063. Jordan, SW; Khavanin, N; Kim, JY. Seroma in Prosthetic Breast Reconstruction. Plast Reconstr Surg, 2016 137(4), 1104-1116. Becker, H; Klimczak, J. Aspiration of Periprosthetic Seromas Using the Blunt SeromaCath. Plast Reconstr Surg, 2016 137(2), 473-475. Sajid, MS; Hutson, K; Kalra, L; Bonomi, R. The role of fibrin glue instillation under skin flaps in the prevention of seroma formation and related morbidities following breast and axillary surgery for breast cancer: a meta-analysis. J Surg Oncol, 2012 106(6), 783-795. Brennan, MJ; DePompolo, RW; Garden, FH. Focused review: postmastectomy lymphedema. Arch Phys Med Rehabil, 1996 77(3 Suppl), S74-S80. Ridings, P; Bucknall, TE. Modern trends in breast cancer therapy: towards less lymphoedema? Eur J Surg Oncol, 1998 24(1), 21-22. Stanton, AW; Levick, JR; Mortimer, PS. Chronic arm edema following breast cancer treatment. Kidney Int Suppl, 1997 59, S76-S81. Brennan, MJ. Lymphedema following the surgical treatment of breast cancer: a review of pathophysiology and treatment. J Pain Symptom Manage, 1992 7(2), 110-116. Brorson, H. Liposuction gives complete reduction of chronic large arm lymphedema after breast cancer. Acta Oncol, 2000 39(3), 407-420. Leal, NF; Carrara, HH; Vieira, KF; Ferreira, CH. Physiotherapy treatments for breast cancer-related lymphedema: a literature review. Rev Lat Am Enfermagem, 2009 17(5), 730-736. Schmitz, KH. Balancing lymphedema risk: exercise versus deconditioning for breast cancer survivors. Exerc Sport Sci Rev, 2010 38(1), 17-24. Casley-Smith, JR; Boris, M; Weindorf, S; Lasinski, B. Treatment for lymphedema of the arm – The Casley-Smith method: a noninvasive method produces continued reduction. Cancer, 1998 83(12 Suppl American), 2843-2860. Kasseroller, RG. The Vodder School: the Vodder method. Cancer, 1998 83(12 Suppl American), 2840-2842. Moore, MM; Freeman, MG. Fibrin sealant in breast surgery. J Long Term Eff Med Implants, 1998 8(2), 133-142. Han, C; Yang, B; Zuo, WS; Zheng, G; Yang, L; Zheng, MZ. The Feasibility and Oncological Safety of Axillary Reverse Mapping in Patients with Breast Cancer: A Systematic Review and Meta-Analysis of Prospective Studies. PLoS One, 2016 11(2), e0150285. 268 [914] [915] [916] [917] [918] [919] [920] [921] [922] [923] [924] [925] [926] [927] References Seyednejad, N; Kuusk, U; Wiseman, SM. Axillary reverse lymphatic mapping in breast cancer surgery: a comprehensive review. Expert Rev Anticancer Ther, 2014 14(7), 771-781. Noguchi, M. Axillary reverse mapping for breast cancer. Breast Cancer Res Treat, 2010 119(3), 529-535. Doscher, ME; Schreiber, JE; Weichman, KE; Garfein, ES. Update on Postmastectomy Lymphedema Management. Breast J, 2016 22(5), 553-560. Asdourian, MS; Skolny, MN; Brunelle, C; Seward, CE; Salama, L; Taghian, AG. Precautions for breast cancer-related lymphoedema: risk from air travel, ipsilateral arm blood pressure measurements, skin puncture, extreme temperatures, and cellulitis. Lancet Oncol, 2016 17(9), e392-e405. Gebruers, N; Tjalma, WA. Clinical feasibility of Axillary Reverse Mapping and its influence on breast cancer related lymphedema: a systematic review. Eur J Obstet Gynecol Reprod Biol, 2016 200, 117-122. Miranda Garcés, M; Mirapeix, R; Pons, G; Sadri, A; Masià, J. A comprehensive review of the natural lymphaticovenous communications and their role in lymphedema surgery. J Surg Oncol, 2016 113(4), 374-380. Moulds, JE; Berg, CD. Radiation therapy and breast reconstruction. Radiat Oncol Investig, 1998 6(2), 81-89. Delfino, S; Brunetti, B; Toto, V; Persichetti, P. Burn after breast reconstruction. Burns, 2008 34(6), 873-877. Lee, KT; Mun, GH. Effects of Obesity on Postoperative Complications After Breast Reconstruction Using Free Muscle-Sparing Transverse Rectus Abdominis Myocutaneous, Deep Inferior Epigastric Perforator, and Superficial Inferior Epigastric Artery Flap: A Systematic Review and Meta-analysis. Ann Plast Surg, 2016 76(5), 576-584. Monteiro, M. Physical therapy implications following the TRAM procedure. Phys Ther, 1997 77(7), 765-770. Wu, PS; Winocour, S; Jacobson, SR. Red breast syndrome: a review of available literature. Aesthetic Plast Surg, 2015 39(2), 227-230. Israeli, R. Complications of acellular dermal matrices in breast surgery. Plast Reconstr Surg, 2012 130(5 Suppl 2), 159S-172S. Ho, G; Nguyen, TJ; Shahabi, A; Hwang, BH; Chan, LS; Wong, AK. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg, 2012 68(4), 346356. Handel, N. The double-bubble deformity: cause, prevention, and treatment. Plast Reconstr Surg, 2013 132(6), 1434-1443. References [928] [929] [930] [931] [932] [933] [934] [935] [936] [937] [938] [939] [940] [941] 269 Park, AJ; Black, RJ; Watson, AC. Silicone gel breast implants, breast cancer and connective tissue disorders. Br J Surg, 1993 80(9), 1097-1100. Rohrich, RJ; Clark, CP 3rd. Controversy over the silicone gel breast implant: current status and clinical implications. Tex Med, 1993 89(9), 52-58. Nemecek, JA; Young, VL. How safe are silicone breast implants? South Med J, 1993 86(8), 932-944. Bridges, AJ; Vasey, FB. Silicone breast implants. History, safety, and potential complications. Arch Intern Med, 1993 153(23), 2638-2644. Gerszten, PC. A formal risk assessment of silicone breast implants. Biomaterials, 1999 20(11), 1063-1069. Balk, EM; Earley, A; Avendano, EA; Raman, G. Long-Term Health Outcomes in Women with Silicone Gel Breast Implants: A Systematic Review. Ann Intern Med, 2016 164(3), 164-175. Colombo, G; Ruvolo, V; Stifanese, R; Perillo, M; Garlaschi, A. Prosthetic breast implant rupture: imaging – Pictorial essay. Aesthetic Plast Surg, 2011 35(5), 891-900. Samuels, JB; Rohrich, RJ; Weatherall, PT; Ho, AM; Goldberg, KL. Radiographic diagnosis of breast implant rupture: current status and comparison of techniques. Plast Reconstr Surg, 1995 96(4), 865-877. Chung, KC; Greenfield, ML; Walters, M. Decision-analysis methodology in the work-up of women with suspected silicone breast implant rupture. Plast Reconstr Surg, 1998 102(3), 689-695. Burkhardt, BR. Capsular contracture: hard breasts, soft data. Clin Plast Surg, 1988 15(4), 521-532. Barnsley, GP; Sigurdson, LJ; Barnsley, SE. Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: a meta-analysis of randomized controlled trials. Plast Reconstr Surg, 2006 117(7), 2182-2190. Potter, EH; Rohrich, RJ; Bolden, KM. The role of silicone granulomas in recurrent capsular contracture: a review of the literature and an approach to management. Plast Reconstr Surg, 2013 131(6), 888e-895e. Wan, D; Rohrich, RJ. Revisiting the Management of Capsular Contracture in Breast Augmentation: A Systematic Review. Plast Reconstr Surg, 2016 137(3), 826-841. Chong, SJ; Deva, AK. Understanding the Etiology and Prevention of Capsular Contracture: Translating Science into Practice. Clin Plast Surg, 2015 42(4), 427436. 270 [942] [943] [944] [945] [946] [947] [948] [949] [950] [951] [952] [953] [954] [955] [956] References Ajdic, D; Zoghbi, Y; Gerth, D; Panthaki, ZJ; Thaller, S. The Relationship of Bacterial Biofilms and Capsular Contracture in Breast Implants. Aesthet Surg J, 2016 36(3), 297-309. Freedman, AM; Jackson, IT. Infections in breast implants. Infect Dis Clin North Am, 1989 3(2), 275-287. Washer, LL; Gutowski, K. Breast implant infections. Infect Dis Clin North Am, 2012 26(1), 111-125. Gudi, VS; Julian, C; Bowers, PW. Pyoderma gangrenosum complicating bilateral mammaplasty. Br J Plast Surg, 2000 53(5), 440-441. Barr, SP; Topps, AR; Barnes, NL; Henderson, J; Hignett, S; Teasdale, RL; McKenna, A; Harvey, JR; Kirwan, CC; Northwest Breast Surgical Research Collaborative. Infection prevention in breast implant surgery – A review of the surgical evidence, guidelines and a checklist. Eur J Surg Oncol, 2016 42(5), 591603. Yoshida, SH; Swan, S; Teuber, SS; Gershwin, ME. Silicone breast implants: immunotoxic and epidemiologic issues. Life Sci, 1995 56(16), 1299-1310. Goren, I; Segal, G; Shoenfeld, Y. Autoimmune/inflammatory syndrome induced by adjuvant (ASIA) evolution after silicone implants. Who is at risk? Clin Rheumatol, 2015 34(10), 1661-1666. Walsh, FW; Solomon, DA; Espinoza, LR; Adams, GD; Whitelocke, HE. Human adjuvant disease. A new cause of chylous effusions. Arch Intern Med, 1989 149(5), 1194-1196. Seleznick, MJ; Martinez-Osuna, P; Espinoza, LR; Vasey, FB. Is silicone associated with connective tissue disease? J Fla Med Assoc, 1991 78(2), 85-87. Houpt, KR; Sontheimer, RD. Autoimmune connective tissue disease and connective tissue disease-like illnesses after silicone gel augmentation mammoplasty. J Am Acad Dermatol, 1994 31(4), 626-642. Shons, AR; Schubert, W. Silicone breast implants and immune disease. Ann Plast Surg, 1992 28(5), 491-499. Hajdu, SD; Agmon-Levin, N; Shoenfeld, Y. Silicone and autoimmunity. Eur J Clin Invest, 2011 41(2), 203-211. Brinton, LA; Brown, SL. Breast implants and cancer. J Natl Cancer Inst, 1997 89(18), 1341-1349. Van Natta, BW; Thurston, JB; Moore, TS. Silicone breast implants – Is there cause for concern? Indiana Med, 1990 83(3), 184-185. Stivala, A; Libra, M; Stivala, F; Perrotta, R. Breast cancer risk in women treated with augmentation mammoplasty (review). Oncol Rep, 2012 28(1), 3-7. References [957] [958] [959] [960] [961] [962] [963] [964] [965] [966] [967] [968] [969] [970] 271 Tang, SS; Gui, GP. A review of the oncologic and surgical management of breast cancer in the augmented breast: diagnostic, surgical and surveillance challenges. Ann Surg Oncol, 2011 18(8), 2173-2181. Kim, B; Roth, C; Young, VL; Chung, KC; van Busum, K; Schnyer, C; Mattke, S. Anaplastic large cell lymphoma and breast implants: results from a structured expert consultation process. Plast Reconstr Surg, 2011 128(3), 629-639. Jewell, M; Spear, SL; Largent, J; Oefelein, MG; Adams, WP Jr. Anaplastic large T-cell lymphoma and breast implants: a review of the literature. Plast Reconstr Surg, 2011 128(3), 651-661. Bizjak, M; Selmi, C; Praprotnik, S; Bruck, O; Perricone, C; Ehrenfeld, M; Shoenfeld, Y. Silicone implants and lymphoma: The role of inflammation. J Autoimmun, 2015 65, 64-73. Rupani, A; Frame, JD; Kamel, D. Lymphomas Associated with Breast Implants: A Review of the Literature. Aesthet Surg J, 2015 35(5), 533-544. Clemens, MW; Miranda, RN; Butler, CE. Breast Implant Informed Consent Should Include the Risk of Anaplastic Large Cell Lymphoma. Plast Reconstr Surg, 2016 137(4), 1117-1122. Clemens, MW; Miranda, RN. Coming of Age: Breast Implant-Associated Anaplastic Large Cell Lymphoma after 18 Years of Investigation. Clin Plast Surg, 2015 42(4), 605-613. Clemens, MW; Horwitz, SM. NCCN Consensus Guidelines for the Diagnosis and Management of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Aesthet Surg J, 2017 37(3), 285-289. Myung, Y; Heo, CY. Relationship between Obesity and Surgical Complications after Reduction Mammaplasty: A Systematic Literature Review and MetaAnalysis. Aesthet Surg J, 2017 37(3), 308-315. Howard, PS; Gardner, PM; Vasconez, LO; Core GB. Complications in endoscopic plastic surgery. Clin Plast Surg, 1995 22(4), 791-796. Michot, A; Alet, JM; Pélissier, P; Grolleau-Raoux, JL; Bodin, F; Chaput, B. Morbidity in combined-procedure associating abdominoplasty and breast surgery: A systematic review. Ann Chir Plast Esthet, 2016 61(1), e9-e19. Dean, C. The emotional impact of mastectomy. Br J Hosp Med, 1988 39(1), 3032, 36, 38-39. Kiebert, GM; de Haes, JC; van de Velde, CJ. The impact of breast-conserving treatment and mastectomy on the quality of life of early-stage breast cancer patients: a review. J Clin Oncol, 1991 9(6), 1059-1070. Massie, MJ; Holland, JC. Psychological reactions to breast cancer in the pre- and post-surgical treatment period. Semin Surg Oncol, 1991 7(5), 320-325. 272 [971] [972] [973] [974] [975] [976] [977] [978] [979] [980] [981] [982] [983] [984] [985] References Maguire, P. Breast conservation versus mastectomy: psychological considerations. Semin Surg Oncol, 1989 5(2), 137-144. Fernandes-Taylor, S; Adesoye, T; Bloom, JR. Managing psychosocial issues faced by young women with breast cancer at the time of diagnosis and during active treatment. Curr Opin Support Palliat Care, 2015 9(3), 279-284. Baider, L. Psychological intervention with couples after mastectomy. Support Care Cancer, 1995 3(4), 239-243. Sheppard, LA; Ely, S. Breast cancer and sexuality. Breast J, 2008 14(2), 176181. Thors, CL; Broeckel, JA; Jacobsen, PB. Sexual functioning in breast cancer survivors. Cancer Control, 2001 8(5), 442-448. Lim, CC; Devi, MK; Ang, E. Anxiety in women with breast cancer undergoing treatment: a systematic review. Int J Evid Based Healthc, 2011 9(3), 215-235. McLaughlin, JK; Lipworth, L; Tarone, RE. Suicide among women with cosmetic breast implants: a review of the epidemiologic evidence. J Long Term Eff Med Implants, 2003 13(6), 445-450. Sarwer, DB; Brown, GK; Evans, DL. Cosmetic breast augmentation and suicide. Am J Psychiatry, 2007 164(7), 1006-1013. McLaughlin, JK; Wise, TN, Lipworth L. Increased risk of suicide among patients with breast implants: do the epidemiologic data support psychiatric consultation? Psychosomatics, 2004 45(4), 277-280. Schreiber, KL; Kehlet, H; Belfer, I; Edwards, RR. Predicting, preventing and managing persistent pain after breast cancer surgery: the importance of psychosocial factors. Pain Manag, 2014 4(6), 445-459. Saxena, AK; Kumar, S. Management strategies for pain in breast carcinoma patients: current opinions and future perspectives. Pain Pract, 2007 7(2), 163177. Vadivelu, N; Schreck, M; Lopez, J; Kodumudi, G; Narayan, D. Pain after mastectomy and breast reconstruction. Am Surg, 2008 74(4), 285-296. Couceiro, TC; Menezes, TC; Valênça, MM. Post-mastectomy pain syndrome: the magnitude of the problem. Rev Bras Anestesiol, 2009 59(3), 358-365. Stanley, SS; Hoppe, IC; Ciminello, FS. Pain control following breast augmentation: a qualitative systematic review. Aesthet Surg J, 2012 32(8), 964972. Amaya, F; Hosokawa, T; Okamoto, A; Matsuda, M; Yamaguchi, Y; Yamakita, S; Taguchi, T; Sawa, T. Can acute pain treatment reduce postsurgical comorbidity after breast cancer surgery? A literature review. Biomed Res Int, 2015 2015, 641508. References [986] 273 Wijayasinghe, N; Andersen, KG; Kehlet, H. Neural blockade for persistent pain after breast cancer surgery. Reg Anesth Pain Med, 2014 39(4), 272-278. [987] Andersen, KG; Kehlet, H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain, 2011 12(7), 725-746. [988] Vila, H Jr; Liu, J; Kavasmaneck, D. Paravertebral block: new benefits from an old procedure. Curr Opin Anaesthesiol, 2007 20(4), 316-318. [989] Naccache, N; Jabbour, H; Nasser-Ayoub, E; Abou Zeid, H; Naja, Z. Regional analgesia and breast cancer surgery. J Med Liban, 2009 57(2), 110-114. [990] Chen, X; Lu, P; Chen, L; Yang, SJ; Shen, HY; Yu, DD; Zhang, XH; Zhong, SL; Zhao, JH; Tang, JH. Perioperative propofol-paravertebral anesthesia decreases the metastasis and progression of breast cancer. Tumour Biol, 2015 36(11), 8259-8266. [991] Tahiri, Y; Tran, DQ; Bouteaud, J; Xu, L; Lalonde, D; Luc, M; Nikolis, A. General anaesthesia versus thoracic paravertebral block for breast surgery: a meta-analysis. J Plast Reconstr Aesthet Surg, 2011 64(10), 1261-1269. [992] Koumanis, DJ; Colque, A; Eisemann, ML; Smith, J. Breast surgery under local anesthesia: second-stage implant exchange, nipple flap reconstruction, and breast augmentation. Clin Plast Surg, 2013 40(4), 583-591. [993] De Gregori, M; Diatchenko, L; Belfer, I; Allegri, M. OPRM1 receptor as new biomarker to help the prediction of post mastectomy pain and recurrence in breast cancer. Minerva Anestesiol, 2015 81(8), 894-900. [994] Cheng, GS; Ilfeld, BM. A review of postoperative analgesia for breast cancer surgery. Pain Manag, 2016 6(6), 603-618. [995] Fujii, Y. Prophylaxis of postoperative nausea and vomiting in patients scheduled for breast surgery. Clin Drug Investig, 2006 26(8), 427-437. [996] Fujii, Y. Management of postoperative nausea and vomiting in women scheduled for breast cancer surgery. J Anesth, 2011 25(6), 917-922. [997] Arsalani-Zadeh, R; ElFadl, D; Yassin, N; MacFie, J. Evidence-based review of enhancing postoperative recovery after breast surgery. Br J Surg, 2011 98(2), 181-196. [998] Rutgers, EJ. Guidelines to assure quality in breast cancer surgery. Eur J Surg Oncol, 2005 31(6), 568-576. [999] Kaufman, CS; Landercasper, J. Can we measure the quality of breast surgical care? Ann Surg Oncol, 2011 18(11), 3053-3060. [1000] Birido, N; Geraghty, JG. Quality control in breast cancer surgery. Eur J Surg Oncol, 2005 31(6), 577-586. About the Authors Ciro Comparetto, MD Division of Obstetrics and Gynecology, Azienda USL Toscana Centro, City Hospital, Prato, Italy Email: [email protected] Franco Borruto, MD Consultant in Health Policy of the Government of Monaco, Principality of Monaco Email: [email protected] Index A B adenocarcinoma, 73, 183, 230 adenoma, 39, 40, 220 adipose tissue, 149, 175, 186, 253 adjunctive therapy, 35, 154 adolescent female, 35, 45, 218 adverse effects, 15, 37, 151, 191, 199 aesthetic, x, xii, 9, 13, 34, 36, 55, 66, 78, 117, 121, 139, 142, 145, 148, 149, 150, 152, 155, 157, 158, 160, 162, 164, 165, 169, 177, 178, 179, 185, 190, 227, 232, 246, 256, 257, 260 aesthetic surgery, xi, xii, 162, 177 amputation, 2, 6, 14 analgesic, 201, 203, 205 anastomosis, 144, 147 anatomy, 79, 99, 141, 144, 147, 163, 164, 168, 190, 218, 234 anesthetics, 10, 22 angiogenesis, 12, 64, 175, 186, 266 angiography, 147, 252 angiosarcoma, 51, 72 artery (ies), 14, 141, 144, 146, 147, 148, 251, 252 aspiration, viii, xiii, 17, 18, 19, 20, 22, 24, 26, 28, 31, 32, 40, 41, 44, 90, 92, 174, 182, 213, 215, 216, 217, 234, 238, 245, 265 asymptomatic, 25, 26, 32, 53, 62, 181, 192 axillary lymph nodes, ix, xv, 238, 239, 242 benign, x, xiv, xv, xvi, xvii, 10, 18, 19, 20, 21, 22, 26, 28, 31, 32, 33, 34, 37, 39, 41, 42, 43, 44, 45, 46, 47, 50, 51, 52, 53, 54, 58, 70, 72, 73, 85, 89, 90, 91, 92, 93, 96, 97, 100, 102, 118, 120, 130, 217, 218, 219, 221, 224, 239 benign tumors, 35, 43, 218 bilateral, 10, 34, 48, 53, 62, 66, 67, 125, 129, 131, 132, 145, 155, 247, 248, 270 biopsy, v, viii, xiii, xiv, xv, xvi, 6, 9, 17, 18, 20, 21, 22, 24, 25, 26, 27, 28, 33, 34, 37, 44, 45, 46, 47, 49, 50, 53, 54, 61, 67, 69, 75, 77, 85, 86, 87, 88, 90, 91, 92, 93, 96, 100, 102, 104, 105, 119, 120, 130, 173, 181, 182, 185, 213, 214, 215, 216, 217,222, 234, 235, 236, 237, 238, 239, 240, 241, 242, 243, 244, 245, 246, 265, 266 body image, 130, 150, 152, 157, 161, 177, 198, 199, 255 body mass index (BMI), 161 BRCA mutations, 126, 135 breast augmentation, xi, 15, 16, 35, 117, 149, 158, 159, 165, 166, 168, 170, 173, 174, 175, 177, 178, 185, 190, 193, 194, 200, 261, 262, 263, 264, 269, 272, 273 breast carcinoma, 9, 18, 21, 22, 40, 44, 48, 49, 53, 70, 73, 75, 76, 112, 128, 153, 215, 230, 231, 233, 239, 241, 245, 247, 272 breast lumps, 44, 52, 53, 221 breast mass, 17, 18, 19, 22, 26, 31, 34, 42, 43, 51, 90, 96, 100, 118, 214, 215, 238 278 Index breast self-examination, xiv, 34, 67, 123, 172 breast ultrasound, 28, 90 breastfeeding, 220, 259 C cancer care, xvi, 207 cancer cells, 165, 182 cancer screening, viii, 19, 68, 119, 175 cancer therapy, vii carcinogenesis, 49, 120, 124, 195 carcinoma, ix, xv, 3, 4, 9, 18, 19, 21, 22, 24, 32, 39, 40, 42, 44, 45, 48, 50, 53, 60, 61, 62, 70, 72, 73, 74, 75, 76, 77, 82, 88, 89, 94, 97, 105, 107, 112, 118, 128, 130, 153, 214, 215, 221, 222, 223, 225, 226, 227, 229, 230, 231, 233, 239, 241, 242, 245, 265, 266, 272 cauterization, 6, 14 cell cycle, 12, 124 cellulitis, 184, 266, 268 centrosome, 127 chemoprevention, 27, 120, 125, 127, 132 chemotherapeutic agents, xv, 127, 128 chemotherapy, vii, xvi, 5, 6, 7, 8, 10, 12, 13, 26, 46, 52, 57, 62, 64, 65, 66, 68, 69, 71, 72, 74, 80, 81, 102, 113, 115, 128, 134, 138, 139, 182, 183, 197, 198, 199, 201, 208, 244, 249 clinical symptoms, 42, 171 clinical trials, 5, 8, 9, 11, 12, 13, 51, 59, 91, 105, 109, 115, 117, 127, 149, 174, 212, 226 complications, v, xv, 15, 22, 24, 26, 53, 77, 83, 87, 106, 135, 138, 139, 145, 147, 148, 151, 153, 159, 160, 161, 162, 163, 165, 167, 168, 169, 170, 171, 173, 175, 176, 178, 181, 185, 188, 189, 190, 192, 194, 195, 197, 224, 250, 259, 260, 262, 263, 264, 265, 268, 269, 271 compression, 20, 92, 177, 186, 187 connective tissue, 73, 169, 170, 171, 190, 194, 195, 269, 270 contour, 79, 141, 151, 159, 162, 178, 254 contraceptives, 36, 42 contracture, 15, 16, 139, 142, 150, 154, 159, 166, 167, 168, 169, 170, 173, 175, 178, 190, 191, 192, 193, 194, 269 counseling, xiv, 22, 35, 125, 126, 129, 130, 133, 192, 197, 198, 247 cure, xv, xvi, 1, 9, 44, 57, 58, 59, 73, 77, 81, 93, 101, 140, 183 cyst, 18, 26, 28, 31, 32, 44, 215 cystic duct, 45 cytokines, 76 cytology, 17, 22, 24, 25, 26, 40, 51, 95, 104, 105, 109, 120, 215, 217, 241, 245 D diagnostic criteria, 50 diagnostic markers, 119 differential diagnosis, 35, 44, 46, 51, 70 disease progression, 203 diseases, vii, xv, xvi, 1, 25, 31, 57, 92, 97, 133, 171, 191, 195 drainage, xv, 28, 41, 90, 97, 100, 102, 108, 110, 111, 115, 146, 175, 185, 186, 187, 188, 193, 239, 264 E ectopic breast, 75, 231 edema, 83, 181, 186, 187, 267 endoscopy, 15, 26, 41, 118, 120, 121 excision, x, 10, 27, 31, 40, 41, 42, 43, 45, 47, 49, 51, 53, 59, 61, 63, 65, 70, 72, 73, 75, 77, 78, 80, 82, 86, 88, 89, 90, 92, 94, 95, 96, 106, 119, 120, 145, 163, 165, 176, 185, 216, 232, 258 F family history, 35, 37, 44, 49, 66, 123, 124, 126, 127, 130, 131, 132, 200, 248 FDA approval, 168, 178 fibroadenoma, 32, 33, 34, 43, 51, 73, 88, 220 fibrocystic disease, 16, 32, 221 Food and Drug Administration (FDA), 15, 106, 167, 169, 170, 172, 178, 190, 197 G genes, 123, 124, 126, 127, 129, 130, 133, 247 genetic alteration, 125, 126 genetic code, 133 genetic factors, 9 Index genetic information, 127 genetic marker, 113 genetic predisposition, 49, 129, 133 genetic testing, xv, 46, 124, 125, 126, 129, 133, 246, 247, 248, 249 genetics, 125, 133, 247 genomics, 247 genotype, 203 gonadotropin-releasing hormone (GnRH), 11 granulomas, 42, 193, 194, 269 gynecomastia, 160 H health care, xiv, xv, xvi, 24, 33, 64, 96, 127, 157, 170, 197, 210, 224 heterogeneity, 8, 12, 55, 56, 76, 88, 107, 128, 139, 151, 205, 247 high-risk women, 26, 119, 125, 132, 215 histological examination, x, xiv, 20, 51, 99 histology, 17, 22, 43, 45, 51, 62, 67, 92, 104, 108, 112 hormonal therapy, xvi, 6, 12, 66 hormones, viii, 7, 8, 10, 11, 21, 27, 35, 36, 74, 76, 93, 182 hyperplasia, 8, 23, 32, 44, 46, 47, 48, 51, 61, 89, 96, 222, 223 hypertrophy, xi, 33, 35, 160, 161, 162, 164, 186, 219, 257 hypoplasia, xi, 38, 40 I iliac crest, 146 imaging, v, xv, 6, 10, 17, 19, 20, 26, 28, 31, 32, 33, 34, 41, 43, 45, 46, 52, 55, 63, 64, 69, 73, 75, 78, 79, 82, 83, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 101, 102, 104, 105, 108, 111, 120, 129, 147, 153, 158, 165, 171, 182, 184, 185, 188, 191, 207, 217, 218, 221, 222, 223, 234, 235, 236, 237, 238, 240, 241, 242, 251, 252, 255, 256, 262, 263, 266, 269 imaging modalities, 26, 63, 96, 104, 147, 171 immune system, 76, 179, 190, 194 immune-therapy, viii implant placement, 15, 160, 166, 193 279 implants, xi, 15, 20, 77, 83, 118, 138, 140, 142, 144, 159, 160, 166, 167, 169, 170, 171, 177, 178, 179, 189, 190, 191, 192, 193, 194, 195, 196, 197, 200, 214, 250, 260, 261, 262, 264, 265, 269, 270, 271, 272 infection, 26, 83, 88, 142, 159, 161, 170, 172, 184, 185, 189, 193 inflammation, 25, 28, 39, 42, 53, 172, 191, 219, 271 informed consent, xii, 80, 126, 157, 197 invasive adenocarcinoma, 23 invasive cancer, 44, 59, 61, 62 ipsilateral, 8, 53, 56, 59, 65, 111, 129, 134, 227, 268 irradiation, xiv, xvi, 6, 10, 57, 59, 61, 63, 65, 67, 69, 72, 78, 80, 81, 82, 94, 101, 102, 140, 233 K keratin, 56 kinetics, 8, 108 L lactation, xi, 4, 32, 35, 163, 173, 263 latissimus dorsi, 14, 38, 78, 137, 145, 154, 251 lesions, xiv, xv, 11, 18, 20, 22, 24, 25, 26, 27, 28, 32, 33, 34, 42, 43, 44, 45, 46, 47, 48, 50, 51, 52, 53, 54, 58, 60, 61, 65, 66, 70, 72, 73, 85, 86, 87, 88, 90, 91, 92, 93, 95, 96, 99, 101, 118, 120, 130, 183, 201, 208, 214, 215, 216, 217, 218, 221, 222, 223, 230, 234, 235, 236, 237, 238 leukocytosis, 184 life expectancy, 126, 155, 201 lifestyle changes, 36 liposuction, 92, 161, 164, 174, 186, 259 lymph node, vii, xv, xvi, 20, 56, 74, 77, 78, 90, 97, 99, 100, 101, 102, 104, 105, 107, 112, 113, 115, 188, 194, 238, 239, 240, 241, 242, 243, 244, 245, 266 lymphadenectomy, v, 97, 106, 184, 244 lymphatic system, 97, 98, 99, 103, 141 lymphedema, 83, 184, 185, 187, 188, 267, 268 lymphocytes, 76 lymphoma, 53, 75, 97, 196, 197, 271 280 Index M macromastia, 33, 35, 218, 219, 260 malignancy, ix, 4, 17, 18, 20, 26, 28, 34, 37, 41, 43, 44, 47, 50, 53, 59, 82, 85, 87, 91, 93, 119, 130, 175 malignant cells, 71, 119 malignant melanoma, 75, 104, 241 malignant tumors, 72, 118 mammogram, 19, 83, 90, 94, 97 mammography, 4, 6, 7, 8, 17, 18, 20, 22, 25, 26, 28, 34, 35, 42, 44, 53, 54, 60, 62, 63, 64, 67, 69, 73, 79, 82, 85, 87, 90, 91, 93, 96, 97, 103, 118, 123, 125, 129, 131, 159, 169, 171, 190, 191, 262, 263 mammoplasty, 16, 36, 38, 43, 83, 133, 157, 158, 159, 161, 162, 164, 165, 166, 170, 172, 176, 177, 178, 190, 191, 192, 194, 196, 197, 257, 259, 262, 263, 270 mammotome, viii, 24, 88 mastalgia, 32, 33, 34, 36, 42, 44, 219 mastectomy, vii, xiv, xv, xvi, 1, 2, 3, 4, 5, 6, 7, 9, 14, 16, 19, 26, 36, 43, 47, 49, 52, 56, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 71, 77, 79, 80, 82, 83, 109, 113, 114, 115, 123, 125, 126, 127, 129, 130, 131, 132, 134, 137, 138, 139, 141, 142, 143, 145,150, 152, 154, 159, 169, 172, 176, 181, 183, 185, 188, 193, 198, 199, 201, 202, 204, 211, 212, 213, 224, 225, 231, 232, 246, 247, 248, 249, 250, 251, 253, 254, 255, 256, 264, 265, 266, 268, 271, 272, 273 mastitis, 4, 33, 39, 42, 220 mastopexy, xi, 16, 158, 160, 162, 163, 164, 166, 175, 245, 246, 257, 258, 260, 264 metastases, vii, 12, 52, 58, 64, 69, 74, 75, 81, 97, 98, 99, 100, 101, 102, 103, 104, 105, 107, 109, 112, 114, 117, 138, 155, 181, 182, 184, 188, 201, 213, 231, 240, 242, 243, 244, 266 morbidity, 6, 10, 14, 18, 37, 44, 55, 56, 68, 69, 72, 80, 88, 96, 97, 103, 104, 105, 108, 110, 113, 117, 121, 142, 144, 146, 147, 154, 157, 175, 176, 181, 185, 189, 198 MRI, 6, 7, 26, 28, 41, 53, 62, 63, 64, 69, 79, 91, 92, 93, 96, 104, 105, 118, 125, 129, 147, 158, 171, 191, 194, 236, 262 N necrosis, 53, 82, 139, 141, 142, 147, 154, 181, 189, 197, 224 nipple-areola complex, xi, 16, 229, 254, 260 nodes, ix, xiv, 12, 77, 98, 99, 100, 101, 102, 105, 108, 110, 111, 115, 188, 239, 240, 241 nodules, 42, 70, 75 non-steroidal anti-inflammatory drugs, 203 normal aging, 179 normal development, 32, 33 O obesity, viii, 145, 189, 197 oophorectomy, 12, 134 opioids, 202, 203 ovarian cancer, 124, 125, 126, 127, 128, 129, 132, 246, 247, 249 P p53, 23, 52, 74, 111, 124, 128 pain, 14, 15, 31, 32, 36, 146, 147, 157, 159, 163, 170, 173, 181, 187, 193, 198, 201, 202, 203, 219, 265, 272, 273 pain management, 202 pathology, xiv, xv, 27, 28, 29, 45, 47, 75, 90, 92, 94, 96, 110, 112, 114, 119, 187, 208, 209, 217, 221, 222, 223, 237, 262 pectoralis major, 38, 58, 143 pharmacological agents, 37 physical therapy, 189 plastic surgery, xii, 78, 117, 138, 139, 149, 150, 153, 159, 167, 169, 173, 177, 179, 185, 197, 198, 258, 271 polymastia, 33, 38 polythelia, 33, 37, 38 prevention, xviii, 8, 11, 14, 27, 40, 46, 67, 123, 126, 130, 131, 132, 134, 176, 185, 187, 190, 193, 204, 205, 212, 246, 248, 267, 268, 269, 270, 273 primary tumor, 56, 65, 68, 75, 95, 98, 111, 113, 153, 182, 228 prophylactic, 27, 47, 49, 67, 77, 113, 123, 125, 127, 130, 131, 132, 134, 155, 183, 204, 246, 247, 248, 266 Index prophylactic surgery, 125, 127, 248 prostheses, 15, 132, 137, 160, 166, 169, 170, 191, 195, 196, 262 prosthesis, xii, 14, 15, 16, 137, 138, 140, 142, 167, 169, 174, 179, 185, 189, 214, 250, 261 ptosis, xi, 160, 161, 162, 164, 175, 257, 258 281 resection, 2, 26, 57, 69, 71, 79, 80, 93, 94, 95, 101, 103, 112, 117, 139, 140, 145, 161, 162, 228, 232, 253 risk factors, viii, xiii, 35, 46, 49, 123, 124, 129, 134, 145, 184, 189, 197, 200, 202, 204, 273 S Q quadrantectomy, x, 6, 9, 66, 79, 139, 212 quality of life, xvii, 57, 63, 68, 69, 76, 78, 126, 131, 132, 133, 152, 154, 157, 161, 177, 198, 199, 201, 255, 258, 271 R radiation, xvi, xvii, 5, 6, 7, 8, 10, 13, 46, 47, 56, 57, 60, 63, 64, 65, 67, 69, 72, 71, 73, 74, 80, 82, 83, 86, 87, 89, 110, 111, 138, 139, 142, 143, 148, 153, 154, 183, 188, 197, 229, 230, 233, 249 radiation therapy, xvi, xviii, 6, 7, 8, 10, 56, 57, 60, 63, 64, 68, 69, 73, 80, 138, 140, 148, 184, 188, 229, 230, 233, 249 radical mastectomy, vii, xiv, 2, 3, 4, 5, 6, 7, 9, 56, 58, 64, 66, 77, 130, 143, 198, 211, 212, 225 radiotherapy, viii, 1, 6, 9, 10, 11, 50, 52, 56, 59, 60, 63, 64, 65, 66, 72, 74, 75, 76, 77, 78, 80, 81, 102, 103, 135, 137, 138, 139, 142, 145, 152, 165, 182, 186, 188, 199, 201, 208, 213, 229, 233, 249 reconstruction, v, xi, xvi, 6, 13, 14, 34, 38, 43, 49, 56, 59, 62, 64, 77, 78, 83, 117, 132, 135, 137, 138, 139, 140, 141, 142, 143, 144, 146, 147, 148, 149, 150, 151, 152, 153, 154, 158, 159, 166, 167, 169, 170, 176, 179, 181, 185, 188, 190, 193, 194, 195, 198, 201, 208, 213, 230, 245, 249, 250, 251, 252, 253, 254, 255, 256, 257, 262, 264, 267, 268, 272, 273 recovery, 15, 152, 165, 176, 189, 202, 203, 205, 273 rectus abdominis, 14, 143, 144, 154, 251 recurrence, xv, xvi, 3, 5, 12, 14, 21, 34, 36, 42, 43, 50, 51, 56, 58, 59, 60, 62, 65, 69, 71, 73, 75, 77, 79, 80, 81, 82, 83, 102, 113, 115, 117, 134, 138, 140, 142, 148, 149, 154, 181, 182, 183, 184, 193, 197, 199, 202, 203, 212, 266, 273 reduction mammaplasty, 159, 248, 258, 259 sarcoma, 71, 73, 75, 124, 229 screening, viii, xiii, xiv, 6, 17, 18, 21, 24, 25, 26, 29, 31, 34, 46, 49, 50, 53, 54, 55, 60, 62, 63, 64, 67, 68, 82, 87, 90, 96, 102, 108, 118, 120, 123, 126, 127, 130, 154, 172, 174, 182, 192, 196, 215, 217, 224, 238, 246, 248, 255, 259, 262 sentinel lymph node, xvi, 239, 240, 241, 242, 243, 244, 245 shape, xi, 3, 15, 78, 138, 144, 147, 153, 157, 158, 160, 161, 163, 164, 166, 168, 169, 178, 191, 257 silicone, xi, 15, 16, 83, 138, 140, 142, 159, 167, 169, 170, 171, 172, 174, 177, 178, 179, 190, 192, 193, 194, 195, 214, 261, 262, 264, 265, 269, 270, 271 stem cells, 56, 149, 175, 253, 263 surgical intervention, ix, 35, 41, 55, 60, 76, 173, 221 surgical resection, 9, 10, 12, 56, 71, 79, 119, 120 surgical technique, xi, xvi, 9, 10, 11, 51, 55, 66, 77, 79, 83, 114, 117, 132, 137, 139, 140, 148, 152, 165, 173, 176, 188, 194, 202, 232, 256 survival rate, ix, xvii, 22, 69, 82, 174 survivors, 151, 187, 199, 254, 267, 272 symptoms, viii, xiv, xv, 11, 17, 31, 33, 36, 42, 93, 160, 161, 169, 170, 181, 197, 199 syndrome, 37, 38, 43, 124, 132, 160, 171, 181, 184, 189, 191, 193, 194, 202, 219, 249, 268, 270, 272 T tamoxifen, 8 techniques, x, xv, xvi, xviii, 2, 5, 9, 14, 15, 16, 17, 21, 23, 24, 26, 28, 29, 34, 35, 38, 40, 43, 50, 52, 54, 56, 57, 59, 63, 64, 65, 75, 78, 80, 81, 86, 87, 88, 90, 91, 93, 94, 95, 96, 100, 101, 102, 105, 106, 108, 114, 117, 118, 120, 121, 138, 139, 140, 143, 146, 147, 148, 149, 150, 151, 153, 154, 159, 160, 161, 162, 163, 164, 165, 166, 170, 173, 174, 175, 185, 186, 189, 191, 198, 201, 204, 215, 216, 282 Index 233, 235, 236, 238, 246, 249, 251, 252, 254, 256, 258, 261, 265, 269 therapy, vii, xv, xvi, xviii, 1, 5, 6, 8, 9, 10, 11, 12, 13, 24, 25, 36, 42, 56, 57, 58, 59, 60, 64, 65, 66, 67, 69, 71, 72, 74, 75, 77, 78, 80, 81, 82, 91, 93, 97, 101, 102, 109, 111, 113, 114, 115, 119, 137, 138, 140, 143, 159, 181, 182, 183, 184, 186, 187, 203, 204, 213, 226, 228, 231, 234, 237, 246, 249, 265, 266, 267, 268 treatment, vii, xiii, xiv, xv, xvi, xviii, 2, 3, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 16, 17, 19, 21, 24, 27, 29, 31, 32, 34, 35, 36, 37, 39, 40, 41, 42, 44, 45, 46, 49, 50, 51, 52, 55, 57, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 70, 71, 72, 73, 74, 75, 76, 77, 78, 80, 82, 83, 85, 91, 92, 93, 94, 96, 101, 102, 103, 104, 108, 109, 112, 118, 129, 131, 134, 139, 140, 142, 149, 154, 166, 167, 168, 182, 183, 184, 185, 186, 187, 188, 190, 193, 195, 196, 197, 198, 199, 201, 203, 208, 211, 212, 214, 218, 219, 220, 224, 225, 226, 227, 230, 231, 233, 236, 237, 238, 242, 244, 245, 248, 256, 257, 264, 265, 266, 267, 268, 271, 272, 273 tumor, vii, xiv, xv, 7, 8, 11, 14, 21, 23, 25, 26, 27, 28, 33, 40, 44, 47, 51, 52, 56, 64, 65, 66, 68, 70, 71, 72, 73, 74, 75, 77, 78, 79, 80, 81, 82, 88, 89, 91, 93, 94, 95, 97, 99, 100, 101, 102, 104, 107, 108, 111, 112, 113, 114, 119, 124, 129, 139, 140, 143, 149, 165, 169, 181, 182, 183, 184, 185, 196, 212, 223, 228, 229, 230, 239, 253, 256 tumor cells, xiv, 23, 27, 56, 93, 98, 99, 107, 114, 149, 182, 239 U ultrasonography, 28, 35, 43, 62, 64, 69, 79, 83, 90, 103, 105, 147, 172, 191 ultrasound, viii, 20, 24, 28, 32, 41, 44, 53, 75, 86, 87, 89, 91, 93, 94, 95, 105, 118, 125, 147, 171, 193, 235, 241 V vascular endothelial growth factor (VEGF), 111 vascular system, 148 W World Health Organization (WHO), 41 wound healing, 142, 159, 176 wound infection, 2, 181 Y young women, 74, 118, 126, 163, 199, 218, 272