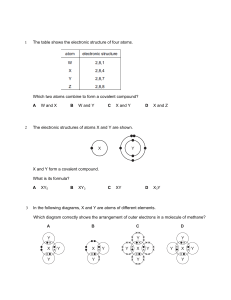
LESSON PLAN SPECIFICATION OF THE SUBJECT Learning Subject : Chemistry Topic Learn : Redox and Electrochemical Grade/Semester : XII/1 Time Allocation : 1 x 25 minutes ACHIEVED COMPETENCY AND THE INDICATORS Standard Competencies : 1. Live and practice the teachings of the religion they hold. 2. Live and practice honest behavior, discipline, responsibility, care (mutual cooperation, cooperation, tolerance, peace), polite, responsive and pro-active and show attitude as part of the solution to various problems in interacting effectively with the social and natural environment and in placing itself as a reflection of the nation in the world association.Understanding, applying, analyzing factual, conceptual, procedural knowledge based on his curiosity about science, technology, art, culture, and humanities with humanity, nationality, state, and civilization insights regarding the causes of phenomena and events, and applying procedural knowledge to the field of study that specific according to their talents and interests to solve problems. 3. Understanding, applying, analyzing and evaluating factual, conceptual, procedural, and metacognitive knowledge based on his curiosity about science, technology, art, culture, and humanities with human, national, state, and civilization insights related to the causes of phenomena and events, and to apply procedural knowledge in specific fields of study according to their talents and interests in solving problems. 4. Cultivate, reason, present, and create in the realm of concrete and abstract domains related to the development of what they learn in school independently and act effectively and creatively, and be able to use methods according to scientific principles. Basic Competencies : 3.3 Evaluate the symptoms or processes that occur in the example of electrochemistry cells (vota cells and electrolysis cells) used in life. 4.3 Create ideas for electrochemistry cell products. Indicators of Competency Achievement : 3.3.1 Identify the meaning of redox reactions. 3.3.2 Determine the oxidation number of a compound. 3.3.3 Balancing the redox reaction equation by the oxidation number method and half the reaction. LEARNING ACTIVITIES Forms of Activities Activity Teacher’s Questions Students Answers Assalamu’alaikum warahmatullah Wa’alaikumussalam warahmatullah wabarakaatuh. Opening Good morning class. Are you doing good today? Students answer the teacher greetings, prays, and condition themselves ready to learn Ok. Before we study, lets pray together. One of you please lead the prayer. wabarakaatuh. Good morning miss. We are fine, alhamdulillah. Ok friends. Before we study, lets pray together. Pray begin. Finish. Ok. Some were absent today? No, miss. Ok. Now please look around you. Is it Yes miss clean? Ok. Then now please take out your books .Ok miss. and prepare the stationery Students and Anyone know what we will learn today? Electrochemistry, miss. Is it right? teacher ask Yes, you are right. Today we are going to Ok, miss. question relate talk about electrochemistry. But, before that to learn. we will talk about redox reactions. First when you were in class X you already Sorry miss. We forgot. got material about redox. Does anyone still remember what is a redox reaction? It’s ok. No problem. We will remember it Ok miss. today. Students listen to Before that, I hope you can identify the learning meaning of redox reactions, determine the objectives and oxidation number of an element, equalize an explanation redox reactions, and be able to cite examples of the benefits of of redox reactions in everyday life after mastering getting this lesson today. Fine, miss. we will try to understand this material. learning material. Main Activities Ok. Look at the picture please. Have you ever seen this picture before? Observing Observe images Yes, it’s true. Anyone know what oxidation is? of rusty apples and nails. Is that a picture of apples and nails undergoing oxidation, miss? Ah, I began to remember, miss. Is oxidation a reaction to a decrease in oxidation number, miss? Yes, very true. Then if redox stands for If oxidation is decreasing, then does the reduction-oxidation. What is reduction? reduction in oxidation number increase? Asking Yah, thats right. Now does anyone remember how to determine the oxidation Asking what is a redox reaction Redox is an increase and decrease reaction of oxidation numbers. Is it true? number of an element? Yes, right. But there is one thing that was and how to missed. In redox reactions other than equalize a redox changes in oxidation numbers, what else reaction. happens? So what is a redox reaction? Electron handover, miss. Redox reactions are electron handover reactions accompanied by changes in oxidation number miss. Then, please pay attention to the following Yes, miss. equation. Is the equation equivalent? Noo, this equation is not equivalent. Try to Not yet, miss. see again. Are the elements on the left and right sides all the same? So what do you think right now? How to make these equations equivalent, miss?/what is the redox relationship with electrochemistry,miss? Data collecting Ok. To answer your question, now please Looking for open your book or your phone. Then please information from look for information on how to balance the various sources redox reaction equation. Ok miss. how to equalize the redox reaction equation. Associate Ok guys. Is it done? Yes, miss. Practice search Then, please answer the questions that are Ok, miss. results. Communicating available on your worksheet. Ok guys. Have you finished? Please one of you presented the result of the discussion. Me, miss. To number one with the half reaction method. The reduction reaction is Present the NO3- → NO2 then the oxidation reaction is results of the Cu → Cu2+. discussion. then because NO2 lacks oxide so H2O is added. After that, because in the left section NO3- lacks as much as 2 hydrogen so 2H+ is added. After that, balance the number of electrons. So the equation becomes NO3- + 2H+ + e̅ → NO2 + H2O. Then, in the other reaction, just equal the number of electrons. So, the equation becomes Cu → Cu2+ + 2̅e. After that, turn it back into one reaction by eliminating electrons. So the reaction equation becomes 2NO3- + 2H+ + Cu → 2NO2 + 2H2O + Cu2+. Yeah, that’s true. Then how to equate it with the method of changing oxidation numbers? Me, miss. First, look for elements that have changed oxidation numbers. That is Nitrogen and Copper. Then, equal the number of electrons there are left and right segments.. It is nitrogen oxidizing by 2 electrons and copper experiencing reduction by 1 electrons. So the equation becomes 2NO3- + Cu → 2NO2 + Cu2+. Because on the right side the charge is +2 while the left side is -2, so it adds 4H+ on the left side. Last, because in the right side it lacks 4 Hydrogen atoms so 2 molecules of H2O are added to the left side. Closing Ok, class. Because the time is almost up. Me, miss. In balancing the redox reaction Anyone want to conclude our material equation there are two methods, namely the today? half reaction method and the oxidation number change method. Yeah, it’s true. Before closing our study No, miss. today, are there any questions? Because there is no question, please oen Ok, miss. Wa’alaikumussalam page 30. There are some questions you can warahmatullah wabarakaatuh. Thank you, do for practice . and don’t forget to learn miss. about electrochemistry for oue material supplies next week. See you next week, Wassalamu’alaikum warahmatullah wabarakaatuh.