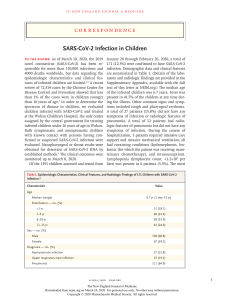
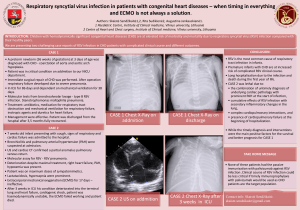
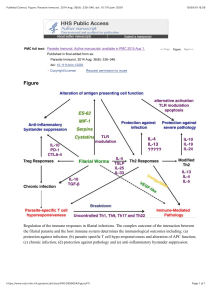
Uploaded by
common.user55459
Optimal Control Model for Chronic Chlamydia Treatment with Antibiotic and Tryptophan
advertisement

Applied Mathematics and Computation 375 (2020) 124899 Contents lists available at ScienceDirect Applied Mathematics and Computation journal homepage: www.elsevier.com/locate/amc An optimal control model of the treatment of chronic Chlamydia trachomatis infection using a combination treatment with antibiotic and tryptophan Morenikeji Deborah Akinlotan a,b,1,2, Daniel G. Mallet a,b, Robyn P. Araujo a,b,∗ a b School of Mathematical Sciences, Queensland University of Technology, 2 George Street, Brisbane, Queensland 4000, Australia Institute of Health and Biomedical Innovation, 60 Musk Avenue, Kelvin Grove, Queensland 4059, Australia a r t i c l e i n f o Article history: Received 12 July 2019 Revised 14 October 2019 Accepted 28 October 2019 Available online 8 February 2020 Keywords: Persistent Chlamydia trachomatis infection Reactivation of latent pathogen Combination therapy Levo-1-methyl tryptophan Optimal control Pontryagins maximum principle a b s t r a c t We develop a mathematical model of within-host Chlamydia dynamics to investigate the effects of different treatment combinations on the within-host dynamics of chronic genital chlamydial infections characterised by the presence of IFN-γ -induced chlamydial persistence. Our modelling framework is informed by recent studies which have demonstrated that tryptophan and some of its biosynthetic analogues are able to both reverse and inhibit IFN-γ -induced chlamydial persistence. Our aim is to find the optimal combination of treatments/drugs that will minimise the systemic cost of two controls/treatments, the concentration of extracellular Chlamydia, infected epithelial host cells, and importantly the development of chlamydial persistence. Pontryagin’s Maximum Principle is used to characterise the optimal controls and the resulting optimal control system is numerically solved. Optimal control solutions that eradicate the pathogen and its persistence are demonstrated. Our numerical results indicate that the optimal way to clear and truncate the progression of a chronic Chlamydia infection may be the administration of a combination therapy that is bacteriostatic to chlamydial particles and that concurrently inhibits and reverses chlamydial persistence, using a cocktail of tryptophan supplementation and levo-1-methyl tryptophan. Our approach provides a framework for the design of new therapeutic regimens and guidelines for the treatment of chronic chlamydial infections. © 2019 Elsevier Inc. All rights reserved. 1. Introduction Chlamydia trachomatis is a Gram-negative obligate intracellular bacterial pathogen that infects the human genital tract and ocular epithelium [1–3]. It is the most commonly reported bacterial STI worldwide [4–6]. The control of the incidence of genital C. trachomatis infection continues to present as a major public health challenge [7]. C. trachomatis genital infection, often referred to as the ‘silent epidemic’, is asymptomatic in 85% of infected women, and 40% of infected men. Consequently, it is commonly undiagnosed and untreated [4,6]. Its sequelae is more severe in women, as they develop serious health ∗ Corresponding author at: School of Mathematical Sciences, Queensland University of Technology, 2 George Street, Brisbane, Queensland 40 0 0, Australia. E-mail addresses: [email protected] (M.D. Akinlotan), [email protected] (D.G. Mallet), [email protected] (R.P. Araujo). 1 The research of this author was supported by QUT (Higher Degree Research tuition sponsorship), Graduate Women Queensland (Freda Bage fellowship), and the Schlumberger Foundation (Faculty for the Future women fellowship). 2 Present address: Queensland Institute of Medical Research Berghofer, 300 Herston Road, Herston, Queensland 4006, Australia. https://doi.org/10.1016/j.amc.2019.124899 0 096-30 03/© 2019 Elsevier Inc. All rights reserved. 2 M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 problems such as chronic pelvic pain, sterility, and cervicitis. Untreated C. trachomatis plays a crucial causative role in severe sequelae such as pelvic inflammatory disease (PID), tubal factor infertility (TFI), life threatening ectopic pregnancy, and sepsis [1,6–9]. Chlamydia displays a unique and complex biphasic developmental cycle involving eukaryotic cells. Within host, they appear in two distinctive morphological forms: the extracellular, metabolically inert, infectious form, known as the elementary body (EB) and the intracellular, metabolically active, and replicative form, known as the reticulate body (RB). Infection commences with the attachment of the EB to the host eukaryotic cell surface; followed by its internalisation; differentiation into the RB forms; repeated binary fission of RBs; asynchronous de-differentiation to EBs; and eventual lysis of the infected host cell at the end of the chlamydial developmental cycle (CDC) [2,9–12]. Genital Chlamydia infection is treatable with antibiotics. Treatment guidelines for uncomplicated urogenital C. trachomatis infection recommend the first line of treatment to be a single 1g oral dose of azithromycin [13]. Another commonly recommended regimen is the twice daily 100 mg of doxycycline taken for 7 days, but this regimen is less preferred due to patient compliance issues [5,14]. These antibiotics exert their antimicrobial activity on Chlamydia through the inhibition of its protein synthesis thereby stalling its growth. Thus they are only bactriostatic on Chlamydia [15,16]. Despite the fact that Chlamydia infection is treatable, and the host immune system initiates an inflammatory response during a primary infection, C. trachomatis still persists asymptomatically in many individuals [2]. Chlamydial persistence is a reversible state in which Chlamydia exists in a viable but non-cultivable form, resulting in a long-term association between Chlamydia and the infected host cell [11,17]. These non-cultivable chlamydial forms, which are developed in the presence of some inducers, are morphologically large, non-infectious, non-replicating, and aberrant RBs, and are commonly referred to as the “persistent” form of Chlamydia [2,8,11,18]. In the presence of growth inhibitors, such as IFN-γ , nutrient deprivation, iron deficiency, monocyte infection, phage infection, penicillin, and some other antibiotics, the CDC of some of the intracellular Chlamydia (RB form) is altered, thus assuming the persistent form indefinitely [2,11,17,18]. However, once these growth inhibitors or stressors are removed, the persistent bacteria differentiate back into infectious forms [8,18–20]. Chlamydial persistence is also a key contributor to treatment failures [16]. Studies show that even post-treatment, Chlamydia is able to exist in its persistent state which is undetectable by cell culture and immunoassay [21–23]. These persistent forms may allow subclinical progression of persistent Chlamydia infections [16]. Chronic (or recurrent) chlamydial infections, which are consequences of either chlamydial persistence, or repeat infection (re-infection), are more precarious than acute infections [24]. Experimental studies indicate that the presence of the same chlamydial serovar (after a previously cleared infection) suggests chlamydial persistence, while a new serovar suggests reinfection [25]. Human epidemiological studies have also shown that with repeat chlamydial infection comes a higher risk of disease [26]. It has also been suggested that chronic chlamydial infections associated with Chlamydia persistence are less responsive to antibiotic treatment [16,27,28]. The host immune response has been implicated in the development of such chronic infections due to the production of inflammatory cytokines which induce chlamydial persistence [8,28]. Animal models show that there is a rapid and large infiltration of CD4+ and CD8+ T cells, as compared to neutrophils, during repeat oviduct infections [26]. This higher infiltration of cytotoxic cells, in comparison to what happens during a primary infection, leads to a robust inflammatory response [26]. Consequently, the recurrent inflammatory reactions, and other processes which occur during chronic chlamydial stimulatory actions, lead to fibrosis, tissue scarring, and cicatrisation within the organ affected [24,26]. All these have been associated with severe sequelae of the infection [24,25]. Although a single infection can elicit a long-term partial protective immune response or a short-term complete immunity, animal models have revealed no evidence of enhanced immunity as a consequence of multiple exposures [3]. Importantly, it has been reported that protective immunity does not remove the severe sequelae of the infection in the upper genital tract of its host [3]. Protective immunity against chlamydial infections have been identified to be mediated primarily by IFN-γ secreting CD4+ and CD8+ T cells [27]. IFN-γ inhibits bacterial growth by inducing indoleamine 2,3-dioxygenase (IDO), an enzyme regulated by the immune system, which depletes or metabolises L-tryptophan, an essential amino acid needed for bacterial protein biosynthesis [27,29]. This IDO-mediated depletion of intracellular pools of tryptophan starves Chlamydia, which is a tryptophan auxotroph [30], of this essential amino acid, thereby inducing bacteriostasis and leading to the development of persistent Chlamydia [8,27,29]. A rapid re-differentiation (reactivation) of these persistent forms occurs when the pool of tryptophan returns to normal levels [27]. There is an increasing interest in the reactivation of latent forms of infectious diseases that can facilitate viraemia rebound post-treatment interruption [31–34], such as in latent HIV-1 infections, which are untreatable with current antiretroviral drugs [32,35,36], latent Mycobacterium tuberculosis infection [31,34], and in latent herpesvirus infection [33]. Studies show that therapeutic reactivation of these latent viruses, in combination with therapeutic (antiretroviral/antibiotic) treatments may accelerate the removal of latent reservoirs thereby facilitating total disease clearance/remission [32,35,37]. The reversal of latent/persistent forms of Chlamydia has also been studied. In vitro experiments have shown that the addition of 1-DL-Methyl-tryptophan (1-MT), a biosynthetic analog of tryptophan (methylated tryptophan) [38–41], is able to reverse or inhibit ID0-mediated tryptophan metabolism and its other antimicrobial or immunoregulatory functions, by directly permitting the growth of parasites or T cells [29]. An in vitro study by Schmidt et al. [29] reports that IDO-mediated antimicrobial effects caused by tryptophan depletion can be abrogated by the L isomer of 1-MT, 1-levo-methyl tryptophan (1-L-MT) (sometimes written as levo-1-methyl tryptophan (L-1MT)), a specific IDO inhibitor which can accumulate to equilibrium levels needed for adequate inhibition of IDO in vivo [29,30]. They also show that 1-L-MT can reinstate the immunoregulatory effects of IDO [29]. Another in vitro study by Ibana et al. [30] also reports that the use of L-1MT, in an in vitro model of M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 3 IFN-γ -induced chlamydial persistence, delayed the depletion of tryptophan induced by the activity of IFN-γ until the late stage of the CDC. This delay caused a blockage of IFN-γ -induced chlamydial persistence. They also observed that L-1MT reactivates established persistent Chlamydia forms, thereby becoming actively replicating RBs intracellularly. Ibana et al. [30] also report that the addition of L-1MT to their IFN-γ -exposed infected HeLa cell culture does not support the productive replication of Chlamydia intracellularly, as the number of EBs produced by lysing infected cells was significantly reduced. The efficacy of antibiotics (doxycycline in particular) in the clearance of persistent chlamydial particles was also improved. Singla [28] also suggested that a better therapeutic treatment of chronic Chlamydia infection could be achieved, if there is a sufficient supply of tryptophan, at about 48-72 hours post-infection, before, and with antibiotic treatment, which may facilitate the eradication of persistence. It was also suggested that this therapy may be useful even in acute infections as the treatment strategy would increase Chlamydia’s susceptibility to antibiotics, while also decreasing the production of persistent Chlamydia [28]. These results are promising and they point to the fact that an antibiotic and tryptophan combination treatment may facilitate an improved treatment of chronic Chlamydia infections characterised by chlamydial persistence. Thus, we consider a mathematical model of the treatment of chronic Chlamydia infections under these scenarios, using optimal control tools. In this study, we present a deterministic mathematical model of C. trachomatis infection, within-host, with a particular focus on the determination of the optimal scheme(s) (that is, when and how treatment should be initiated) for the treatment of chronic chlamydial infections using combination therapy of antibiotics and tryptophan supplementation (tryptophan or a cocktail of tryptophan and L-1MT). Our work aims to determine optimal treatment strategies that not only minimise the production of free extracellular Chlamydia, but also minimise the production of persistent intracellular Chlamydia by blocking the formation of persistent Chlamydia, and reversing already established persistence into actively replicating Chlamydia forms, for clearance by the immune system and antibiotics. While there are many growth inhibitors/stressors that can induce chlamydial persistence as earlier discussed, this study focuses particularly on the reversal of IFN-γ -induced chlamydial persistence. In Section 2, we develop a model for the optimal control of Chlamydia infection. We also present basic properties of the developed model, such as the positivity of solutions, and the existence and stability of equilibria, in Section 2. A global uncertainty and sensitivity analysis of the basic Chlamydia model’s response variable, the basic reproduction number R0 , to variations in the predictors (input parameters) of the model system is also conducted in Section 2. Using an existence result, we guarantee the existence of an optimal control pair with finite objective functional in Section 3.2. In Section 3.3, we use Pontryagin’s Maximum Principle to characterise the optimal control pair. We present numerical results of simulation of the model system in Section 4. Our conclusions are discussed in Section 5. 2. Formulation and analysis of a within-host model of persistent Chlamydia (No treatment) We develop a mathematical model of the cellular dynamics of Chlamydia and the infected host system, in the absence of treatment, in order to examine its basic properties, thereby establishing a mathematical framework that will be built upon for the investigation of the impact of various treatment strategies for chronic/persistent chlamydial infection. Our mathematical framework extends a model of within-host Chlamydia interactions with the immune system originally proposed by Wilson [42], by introducing an additional compartment comprising a latent (persistent) form that is induced by IFN-γ an established mechanism by which Chlamydia partially eludes the immune system. This gives rise to an infected compartment comprising three distinct components, rather than the two-component infected compartment considered by Wilson [42]. Ordinary differential equations are used to model the cellular dynamics of the interactive processes between extracellular Chlamydia, uninfected epithelial cells, Chlamydia-infected epithelial cells, and Chlamydia-infected epithelial cells within which Chlamydia is in the persistent state. The model describes the role of the humoral and cell-mediated immunity in the course of a Chlamydia infection. We denote by C(t), the concentration of free extracellular Chlamydia, E(t), the concentration of uninfected mucosal epithelial cells, I(t), the concentration of Chlamydia-infected epithelial cells, and IP (t), the concentration of Chlamydia-infected epithelial cells, within which Chlamydia is in the latent/persistent state. We shall refer to IP simply as persistently infected cells. The following system of equations, which we shall refer to as the (untreated) ‘basic Chlamydia (persistence) model’, is proposed: dC = P k2 I − μC − k1CE, dt dE = PE − δE E − k1CE, dt dI = k1CE − γ I − k2 I, dt dIP = γ QI − δP IP , dt (1) (2) (3) (4) with initial conditions C (t0 ) = C0 , E (t0 ) = E0 , I (t0 ) = I0 , and IP (t0 ) = IP0 , where t0 is the initial time. the rate of clearance of extracellular Chlamydia by macrophages - a component of the humoral immunity). However, when u1 > 0, μ = μ + μτ , where μτ is the rate at which tryptophan (either as a supplement or as a cocktail of the supplement and L-1MT) facilitates the reduction in the amount of EBs produced on lysis of infected epithelial cells. 4 M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 Fig. 1. A schematic diagram of the (untreated) ‘basic’ Chlamydia persistence model (1)–(4). The symbols C(t), E(t), I(t), and IP (t) represent the concentrations of extracellular Chlamydia, healthy epithelial cells, infected epithelial cells, and persistently infected epithelial cells, respectively. At a rate of k2 , P Chlamydia are released from infected cells. These extracellular Chlamydia are cleared by macrophages a component of the humoral immunity - at a rate μ. The rate of epithelial cell infection (which may be reduced by antibodies) is denoted by k1 , the rate of production of epithelial cells is denoted by PE , the natural death rates of healthy epithelial cells and persistently infected (epithelial) cells are denoted by δ E and δ P , respectively. We assume that infected cells either burst/lyse (after the maturation of their intracellular chlamydial developmental cycle) or become persistently infected. Thus we do not account for their natural deaths. The rate of clearance of infected cells, due to cell-mediated immunity (predominantly by the secretion of IFN-γ ) is denoted by γ . IFN-γ -induced persistence occurs in a fraction Q (0 < Q < 1) of infected cells (due to the activity of the cell-mediated immunity). A flow chart that describes the above biological process for the basic (untreated) Chlamydia persistence model is illustrated on Fig. 1. 2.1. Basic properties In this section, we present some basic qualitative results for the model system (1)-(4), in order to ascertain that the problem is mathematically and biologically well posed. We first establish that in the dynamical model, all state variables remain non-negative for all time (Appendix A, Lemma A.1). We further establish a biologically feasible region D, which is positively invariant and attracting with respect to the model system (1)–(4), given by D = (C (t ), E (t ), I (t ), IP (t )) ∈ R4+ : C (t ) ≤ n¯1 , E (t ) ≤ E ∗ , I (t ) ≤ n¯2 , IP (t ) = n¯3 , e−μt (5) t t C (0 ) + P k2 0 I (τ )eμτ dτ , n¯2 = e−k2 t I (0 ) + k1 0 C (τ )E (τ )ek2 τ dτ , and n¯3 = e−δP t IP (0 ) + γ Q 0 I (τ )eδP τ dτ with n¯1 = (Appendix A, Lemma A.2). t 2.2. Existence and stability of equilibria In this section, we determine the equilibria of the basic Chlamydia persistence model (1)–(4) and analyse their stability. 2.2.1. Local stability of the Chlamydia-free equilibrium The Chlamydia-free equilibrium (CFE) can be obtained by the setting the right-hand sides of the basic Chlamydia persistence model (1)–(4) to zero and then choosing solutions where C = I = IP = 0. This CFE is given by F0 , F0 = (C ∗ , E ∗ , I∗ , IP∗ ) = (0, Eˆ , 0, 0 ), where Eˆ = PE δE (6) . The linear stability of this equilibrium F0 can be established using the next generation method (as described by van den Driessche and Watmough [43]) on the model system (1)–(4). The class of infectives in the model system (1)–(4) are Chlamydia (C), Chlamydia-infected host cells (I), and persistently infected host cells (IP ), since these three classes facilitate the chronic chlamydial infection process. Thus, the infected subsystem of the model system (1)-(4) is given by Eqs. (1), (3) and (4), that is, (C˙ , I˙, IP˙ ). Hence we sort the model system (1)–(4) so that the first three compartments correspond to the class of infectives, that is (C˙ , I˙, IP˙ , E˙ ). The rate of appearance of new infections in the four compartments, is denoted by F1 , ⎛ ⎞ 0 ⎜k1CE ⎟ F1 = ⎝ , 0 ⎠ 0 (7) while the rate of transfer of each of the interacting species in and out of the four compartments is denoted by V1 , ⎛ ⎞ P k2 I − μC − k1CE ⎜ − ( k2 + γ )I ⎟ V1 = −⎝ . γ QI − δP IP ⎠ PE − δE E − k1CE (8) M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 5 Hence, the matrices of partial derivatives F1 and V1 , for the infected subsystem, are respectively given by ⎛ ∂ F1 ( F0 ) ⎜ ∂C ⎜ ⎜ ∂ F2 ( F0 ) F1 = ⎜ ⎜ ∂C ⎜ ⎝ ∂ F3 ( F0 ) ∂C and ⎛ ∂ V1 ( F0 ) ⎜ ∂C ⎜ ⎜ ∂ V2 ( F0 ) V1 = ⎜ ⎜ ∂C ⎜ ⎝ ∂ V3 ( F0 ) ∂C ∂ F1 ( F0 ) ∂I ∂ F2 ( F0 ) ∂I ∂ F3 ( F0 ) ∂I ⎞ ∂ F1 ( F0 ) ∂ IP ⎟ ⎟ 0 ∂ F2 ( F0 ) ⎟ ⎟ = k1 Eˆ ∂ IP ⎟ ⎟ 0 ∂ F3 ( F0 ) ⎠ ∂ IP ∂ V1 ( F0 ) ∂I ∂ V2 ( F0 ) ∂I ∂ V3 ( F0 ) ∂I ⎞ ∂ V1 ( F0 ) ∂ IP ⎟ ⎟ μ + k1 Eˆ ∂ V2 ( F0 ) ⎟ ⎟= 0 ∂ IP ⎟ ⎟ 0 ∂ V3 ( F0 ) ⎠ ∂ IP The operator V1 −1 is given by V1 −1 = ⎛ k2 + γ ⎜ 0 1 ⎝ ˆ (μ + k1 E )(k2 + γ ) F1V1 −1 = 0 0 0 0 P k2 μ + k1 Eˆ γ Q (μ + k1 Eˆ ) δP 0 1 k1 Eˆ (k2 + γ ) (μ + k1 Eˆ )(k2 + γ ) 0 0 P k1 k2 Eˆ 0 0 0 , 0 (9) −P k2 k2 + γ −γ Q 0 0 . δP (10) ⎞ 0 ⎟ 0 ⎠. (μ + k1 Eˆ )(k2 + γ ) (11) δP 0 0 . 0 (12) Hence, the spectral radius of F1V1 −1 , which is the basic reproduction number R0 of the basic Chlamydia persistence model (1)–(4), is given by R0 = P k1 k2 Eˆ . (μ + k1 Eˆ )(k2 + γ ) (13) By inspecting the basic reproduction number R0 , one can track the contribution of the infected and infectious classes (infected epithelial cells and elementary bodies, respectively) to the infection process. From the expression in (13), it can be seen that the basic reproduction number R0 is the product of the infection rate of healthy epithelial cells by Chlamydia, 1 k1 Eˆ , the number of infectious progenies released by a lysing infected cell, P, the duration of infectiousness of an EB, , ˆ and the proportion of infected cells that survive up to the stage of lysis, k2 k2 +γ μ+ k 1 E . We establish the following result by implementing Theorem 2 of van den Driessche and Watmough [43]. Lemma 2.1. The Chlamydia-free equilibrium (CFE) F0 , of the basic Chlamydia persistence model (1)-(4), is locally stable whenever R0 < 1 and unstable if R0 > 1. Lemma 2.1 implies that when R0 < 1, the in vivo clearance of Chlamydia body forms can be achieved if the initial sizes of the subpopulations of the model (C, E, I, IP ) are in the basin of attraction of the CFE F0 . Importantly, we establish the globally asymptotically stable (GAS) of the CFE when R0 < 1 (Appendix A, Theorem A.3). This is to ensure that the therapeutic effects of an effective Chlamydia infection treatment regimen in an in vivo or in vitro setting system does not depend on either the initial size of Chlamydia body forms or innoculum, respectively, or the initial sizes of other subpopulations of the model (E, I, and IP ). We further show that the basic model (1)–(4) has a unique Chlamydia-present equilibrium (CPE) (Appendix A, Theorem A.4) given by F1 , whenever R0 > 1, where F1 = (C ∗∗ , E ∗∗ , I∗∗ , IP∗∗ ), and (14) PE (k2 (P − 1 ) − γ ) δE C ∗∗ = − , μ ( k2 + γ ) k1 (15) E ∗∗ = (16) μ ( k2 + γ ) , k1 ( k2 ( P − 1 ) − γ ) I∗∗ = PE μδE − , k2 + γ k1 ( k2 ( P − 1 ) − γ ) IP∗∗ = γQ PE μδE − . δP k2 + γ k1 (k2 (P − 1 ) − γ ) (17) (18) 6 M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 Fig. 2. PRCC values for the basic Chlamydia model (1)–(4), using the basic reproduction number R0 (13) as the response variable. Parameter values and ranges used are as in Table 1. As Theorems A.3 and A.4 imply, it suffices to explore therapeutic strategies that can drive the disease outbreak threshold R0 below unity. 2.3. Sensitivity analysis The input parameters and initial conditions for predictor and response variables of mathematical models are often unknown, thereby leading to some degree of uncertainty in their exact measurements or estimates. Thus, there is the need to quantify the degree of confidence in the parameter estimates and experimental data [44]. Using a global uncertainty and sensitivity analysis, we investigate and quantify the uncertainty and sensitivity of the response variable R0 of the basic model system (1)-(4), to variations in the predictors (input parameters) of the model. Our analysis uses the Latin Hypercube Sampling (LHS) and partial rank correlation coefficient (PRCC) with 10 0 0 Monte Carlo simulations per run. The LHS is a stratified Monte Carlo sampling without replacement technique which is efficient for the unbiased simultaneous sampling of predictors in a multi-dimensional parameter space, for the execution of an uncertainty analysis [44–46]. Uncertainty analysis is a method that is very useful for evaluating the variability, that is, prediction imprecision, in a response due to the uncertainty in the determination or selection of its predictor(s) [45,46]. A sensitivity analysis extends an uncertainty analysis by determining the predictors with the most significant impacts on the response. It quantifies how uncertainty in the predictors impact on the model response(s). It does this by ranking the predictors using the calculation of PRCCs for each predictor and the model response(s) [44,46]. The sign (positive or negative) of a predictor’s PRCC indicates the qualitative relationship it was with the response [45,46], while its magnitude illustrates the importance of the uncertainty estimation of the predictor variable is to the variability in the response [46]. Predictors with large PRCCs are deemed the most influential in the model. By examining the scatter plot of a predictor’s PRCC, the monotonicity between it and the response can also be assessed [46]. PRCC values are only useful when there is linearity and monotonicity between predictor(s) and response(s) [44]. We investigate the existence of non-monotonicity between the predictors of the basic model (1)-(4) and the model’s R0 , by examining the scatter plots of their PRCCs (figures not shown). The study show that all the predictors have monotonic relationships with the model’s R0 on a log 10 scale. The predictor with the most significant monotonic relationship with R0 is P, the burst size per infected cells. The use of PRCCs for this global sensitivity analysis is thus considered relevant because of the monotonous relation between the predictors and response. In order to conduct sensitivity and uncertainty analysis on the model system (1)–(4), we implement the sampling and sensitivity analysis methods used in SaSAT (Sampling and Sensitivity Analysis Tools) [45]. Tornado plots are used to illustrate the results of the sensitivity analysis [45]. Fig. 2 is the tornado plot of the PRCC of all the predictor variables of the basic model system (1)-(4). Predictor variables that increase R0 when increased are depicted by bar plots to the right, while those that decrease R0 when increased are depicted by bar plots to the left. Fig. 2 shows that the predictors with the most positive influence on chronic (or persistent) within-host Chlamydia infection dynamics, with their PRCCs bracketed, are P, the burst size per infected cell (0.9982) and k1 , the rate of cell infection M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 7 Table 1 Variables, parameters, values, and ranges used in numerical simulations. Tryp. is tryptophan. ♠: Estimated values. ♣: μτ = 1, and ρ = 13, in the presence of tryptophan and a bacteriostatic agent while μτ = 2, and ρ = 15, in the presence of a cocktail of tryptophan and L-1MT, and a bacteriostatic agent. : The maximum tissue concentrations of tryptophan and the bacteriostatic agent, at the site of infection, are assumed to be less than the highest tissue concentration obtainable (that is, 1). Variables Description Values C E I IP Free extracellular Chlamydia Healthy mucosal epithelial cells Chlamydia-infected epithelial cells Persistently infected epithelial cells C0 = 500 cells/mm3 E0 = 50 0 0 cells/mm3 I0 = 100 cells/mm3 IP0 = 10 0 0 cells/mm3 Burst size per infected cell Rate of cell infection Rate of lysis of infected cells Production rate of mucosal epithelial cells Rate of natural death of epithelial cells Rate of natural death of IP Effectiveness of cell-mediated immunity Effectiveness of humoral immunity (no controls) Reduction of EB production as induced by tryptophan Rate at which Tryp. reverses persistence Fraction of IFN-γ -induced IP Maximum dosage of control u1 (t) Maximum dosage of control u2 (t) 200 0.003 cell/mm3 /day 0.33 day−1 44 cells/mm3 /day 0.25 day−1 0.27 day−1 9 day−1 2 day−1 1♣ day−1 13♣ day−1 0.6 0.9 0.9 Parameters P k1 k2 PE δE δP γ μ μτ ρ Q m1 m2 Ranges [200,500] [42] [0.0005,0.01]♠ [0.33,0.6] [42] [30,60] [42,66] [0.25,0.26] [67,68] [0.25,0.3]♠ [2,10][42] [2,10] [42] [0.5,2]♠ [5,15]♠ (0,1]♠ (0.1278). Other predictors with slightly significant positive impact on the response R0 include k2 , the rate of lysis of infected cells (0.0404) and PE , the production rate of mucosal epithelial cells (0.03612). On the other hand, the predictors with negative influences, though with little significance, on R0 are μ, the effectiveness of the humoral immunity (-0.03084), δ E , the natural mortality rate of epithelial cells (-0.03098), and γ , the effectiveness of cell-mediated immunity (-0.01394). 3. The optimal control problem Optimal control theory is a source of very useful and flexible tools for research activities in optimal therapies in medicine, optimal strategies in economics, and in many other fields of applied sciences [47,48]. It is a powerful mathematical tool that can be used to make decisions involving complex biological situations. Its use has thus been on the rise in epidemiological and biological models [49]. The impacts of optimal control on the spread of infectious diseases have been studied in many epidemiological models (see [50–55] and references within). In particular, several within-host models of infectious diseases have used optimal control to predict optimal therapeutic intervention strategies [50,52,56–58]. However, to the best of our knowledge, no within-host mathematical model has been developed to study optimal intervention strategies for Chlamydia infections. 3.1. Model formulation As highlighted in the introductory Section 1, this investigation of treatment strategies for persistent sexual chlamydial infection is motivated by recent studies of the reactivation of latent pathogens [32,35,36], such as Chlamydia [38–41], which would then allow for further clearance by bacteriostatic/bactericidal agents and the immune system. A treatment/control measure that reduces Q, the fraction of IFN-γ -induced persistent Chlamydia (within infected cells), will lead to fewer infected epithelial cells developing intracellular Chlamydia persistence. Consequently, less inflammatory responses that lead to scarring of the genital tissues will occur. As discussed in Section 1, tryptophan supplement can facilitate the reactivation of persistent Chlamydia [28–30]. Levo-1-methyl tryptophan (L-1MT) can also block the formation of IFN-γ -induced persistent Chlamydia, while also facilitating a reduction in the burst size of lysing infected cells [29,30]. Thus, we introduce a time dependent control variable u1 (t) to capture these therapeutic effects. In order to determine additional optimal control measures that should be put in place for the treatment of a chronic Chlamydia infection, we consider the results of the global sensitivity analysis. These results suggest that an effective treatment/control strategy should reduce a combination of one or more of (i) P, the burst size per infected cell, (ii) k1 , the rate of cell infection, (iii) k2 , the rate of lysis of infected cells, and (iv) PE , the production rate of mucosal epithelial cells. Obviously, it may not be desirable to perturb PE . The results also suggest that an improvement on the host system’s ability to clear the infection (via the effectiveness of the humoral (μ) and cell mediated (γ ) immunities), or the rapid death of epithelial cells (δ E ), though undesirable, will abate the chlamydial infection. A control measure that reduces P will result in the release of fewer infectious progenies (that is, elementary body forms of Chlamydia) by bursting cells, to surrounding epithelial host cells, thereby reducing the multiplicity of the chlamydial 8 M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 infection. Consequently, there will be a reduction in the rate of cell infection, that is, k1 , and the overall infection. Bacteriostatic agents such as antibiotics (azithromycin and doxycycline for example [5,13,14]) are effective at inhibiting the protein synthesis of Chlamydia, thereby stalling their intracellular growth [15,16]. This will have a reduction effect on the rate of infected cell lysis - k2 (since the regular lytic cycle is halted [10]) and the burst size P. Thus, we consider the introduction of a time dependent control variable u2 (t) to capture these therapeutic effects. The ordinary differential equation model proposed below describes the role of the humoral and cell-mediated immunity in the course of a Chlamydia infection, while also capturing the effects of different treatment regimens in the clearance of a Chlamydia infection. We apply techniques of optimal control theory to the resulting system of ordinary differential equations and explore optimal control strategies associated with the different kinds of controls/treatment of chlamydial infections described. The functions u1 and u2 represent two different treatments/drugs. The function u1 , which is actually a proportion, corresponds to the tissue concentration of tryptophan (either as tryptophan supplement or as a cocktail of tryptophan and L-1MT), which facilitates the ‘recovery’ of persistently infected cells by reversing IFN-γ -induced persistence in intracellular Chlamydia (thereby increasing the susceptibility of Chlamydia to antibiotic treatment), while also blocking the intracellular formation of persistent Chlamydia in the presence of L-1MT. The function u2 corresponds to the tissue concentration of bacteriostatic agents (which reduces the concentration of infected cells by blocking the intracellular growth of Chlamydia). Both tissue treatment/drug concentrations are present at the sites of infection. The functions u1 and u2 are bounded Lebesgue integrable functions satisfying 0 ≤ u1 (t) ≤ m1 ≤ 1 and 0 ≤ u2 (t) ≤ m2 ≤ 1, where m1 and m2 are the maximum tissue concentrations of u1 (t) and u2 (t), respectively, at the site(s) of infection. We define the control functions on fixed time intervals since chlamydial infection treatments are administered in finite time. Thus the controls are defined for t0 ≤ t ≤ tf , where t0 is the time of commencement of treatment; tf is the time treatment ends; and for current recommended treatment guidelines, t f − t0 ≤ 7 days. For a treatment that includes both antibiotics, tryptophan and L-1MT, the following system of equations is proposed: dC = (1 − u2 )P k2 I − μC − k1CE, dt dE = PE − δE E − k1CE, dt dI = k1CE + ρ u1 IP − γ I − k2 I, dt dIP = (1 − u1 )γ QI − ρ u1 IP − δP IP , dt (19) (20) (21) (22) with initial conditions C (t0 ) = C0 , E (t0 ) = E0 , I (t0 ) = I0 , and IP (t0 ) = IP0 , where t0 is the initial time. We model two different processes into the parameter μ. When u1 ≡ 0, μ is as described (the rate of clearance of extracellular Chlamydia by macrophages - a component of the humoral immunity). However, when u1 > 0, μ = μ + μτ , where μτ is the rate at which tryptophan (either as a supplement or as a cocktail of the supplement and L-1MT) facilitates the reduction in the amount of EBs produced on lysis of infected epithelial cells. IFN-γ -induced persistence occurs in a fraction Q (0 < Q < 1) of infected cells (due to the activity of cell-mediated immunity). The rate at which tryptophan reverses already formed IFN-γ -induced persistence in intracellular Chlamydia is ρ . We assume that this rate may be higher in the presence of L-1MT. The reduction term 1 − u1 in Eq. (22) reduces or blocks the formation of IFN-γ -induced persistence in the presence of L-1MT only. The reduction term 1 − u2 in Eq. (19) reduces or blocks the production of EBs in the presence of antibiotics. The goal of treatment is to minimise systemic costs of the treatments/drugs to the host over the course of the treatment, while also minimising the concentrations of extracellular Chlamydia, infected host cells, and persistently infected cells present at treatment cessation. Hence, we seek an optimal control pair (u∗1 , u∗2 ), such that J (u∗1 , u∗2 ) = min J (u1 , u2 ), (23) u1 ,u2 ∈ where , the set of admissible controls, is defined as = {(u1 , u2 )|u1 and u2 are Lebesgue measurable, 0 ≤ u1 ≤ m1 , 0 ≤ u2 ≤ m2 , m1 , m2 ≤ 1, t ∈ [t0 , t f ]}. (24) The objective functional to be minimized is J ( u1 , u2 ) = tf t0 A1 2 A2 2 u + u dt + A3C (t f ) + A4 I (t f ) + A5 IP (t f ), 2 1 2 2 (25) where tf is the final time of the therapy, and the positive constant weights A1 , A2 , A3 , A4 , and A5 , measure the relative (balancing) costs of implementing the respective treatments over the period [t0 , tf ]. We suppose that the cost function is a nonlinear function of u1 and u2 , u1 , u2 ∈ L2 . Thus, We assume that the controls are quadratic, which is in line with several other literatures [51,53,57–60]. A A The terms 21 u21 and 22 u22 describe the costs associated with administering the respective treatments/drugs. The terms A3 C(tf ), A4 I(tf ), and A5 IP (tf ) are terminal costs associated with the minimisation of the concentrations of Chlamydia, infected cells, and persistently infected cells, respectively, by the end of the treatment. M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 9 3.2. Existence of an optimal control pair In this section, we show that the existence of an optimal control pair with finite objective functional is guaranteed for our model system (19)–(22). We use an established theorem in Fleming and Rishel [61] (refer to the conditions in III.2.4, Theorem III.4.1, and its corresponding Corollary III.4.1), which gives sufficient conditions for the existence of an optimal control pair, for a given model, and a given objective functional. Theorem 3.1. For the optimal control problem with state Eqs. (19)–(22), there exists an optimal control pair (u∗1 , u∗2 ) ∈ which minimises the objective functional J(u1 , u2 ) in Eq. (25). Proof. To prove this theorem, we show that our optimal control model meets the five conditions given in Theorem III.4.1 [61], being sufficient conditions for the existence of an optimal control pair for our model. 1. To verify condition 1, we refer to Theorem 3.1 by Picard-Lindelöf [62,63]. Based on the theorem, if the solutions of the state Eqs. (19)–(22) are a priori bounded and if the state equations are continuous and Lipschitz-continuous in the state variables, then there is a unique solution corresponding to every admissible control pair in . Since for all (C, E, I, IP ) ∈ D, the model states are bounded below and above (see Section 2.1), then, the solutions to the state equations are bounded. The boundedness of the partial derivatives with respect to the state variables in the state system can be shown directly. The right sides of the state Eqs. (19)–(22) are also continuously differentiable functions of the dependent variables C, E, I, and IP . These demonstrate the fact that the system is locally Lipschitz-continuous with respect to the state variable [64]. Thus, condition 1 is fulfilled. 2. The control set is closed and convex by definition. Hence, condition 2 is fulfilled. 3. The right side of the model system (19)–(22) is Lipschitz-continuous. Thus, it is obviously continuous and bounded. The state Eqs. (19)–(22) are also bilinear in u1 and u2 , hence condition 3 is satisfied. A A 4. The integrand G = 21 u21 + 22 u22 , of the objective functional, has a positive semidefinite Hessian, hence it is convex on the admissible set . Thus condition 4 is satisfied. 5. Let η = min(A1 , A2 ), and θ = 12 η. Then, A1 2 A2 2 u + u 2 1 2 2 1 ≡ η (u21 + u22 ) + σ1 u21 + σ2 u22 2 ≥ θ (u21 + u22 ) G= ≥ θ (u21 + u22 ) − c = θ (|u1 |2 + |u2 |2 ) − c, for any c > 0, where 0 ≤ σ 1 ≤ A1 and 0 ≤ σ 2 ≤ A2 . Note that η, θ ≥ 0. Hence the integrand satisfies the inequality in condition 5, with β = 2. This completes the proof. 3.3. Characterisation of the optimal control pair In this section, we derive the conditions of optimality for the control problem (19)-(25). We use Pontryagin’s Maximum Principle [47], which provides the necessary conditions that an optimal control and corresponding state must satisfy, to convert the optimisation problem (19)-(25) into one of minimising a Hamiltonian H, with respect to the control (u1 (t), u2 (t)) [49, pg 14]. Theorem 3.2. Given optimal controls u∗1 , u∗2 , and solutions C∗ , E∗ , I∗ , and IP∗ of the corresponding state system (19)-(22), that minimise the objective functional (25) over , there exist adjoint variables λC , λE , λI , and λIP satisfying: λ˙C = (μ + k1 E ∗ )λC + k1 E ∗ λE − k1 E ∗ λI , (26) λ˙E = k1C ∗ λC + (δE + k1C ∗ )λE − k1C ∗ λI , (27) λ˙ I = −(1 − u2 )Pk2 λC + (γ + k2 )λI − γ Q (1 − u∗1 (t ))λIP (28) λ˙IP = −ρ u∗1 (t )λI + ρ u∗1 (t )λIP + δP λIP , (29) with transversality conditions λC (t f ) = A3 , λI (t f ) = A4 , λIP (t f ) = A5 , and λE (t f ) = 0. (30) 10 M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 Furthermore, the control functions can be shown to satisfy the following, called the control characterisations: u∗1 (t ) = min max 0, u∗2 (t ) = min max 0, γ QI∗ λIP + ρ IP∗ λIP − ρ IP∗ λI P k2 I∗ λC A2 A1 , m1 , , m2 . Proof. We define the Hamiltonian H (C, E, I, IP , u1 , u2 , λC , λE , λI , λIP ) as H= A1 2 A2 2 u + u + λC ((1 − u2 (t ))P k2 I (t ) − μC (t ) − k1C (t )E (t )) + λE (PE − δE E (t ) − k1C (t )E (t )) 2 1 2 2 + λI (k1C (t )E (t ) + ρ u1 (t )IP (t ) − γ I (t ) − k2 I (t )) + λIP (γ Q (1 − u1 (t ))I (t ) − ρ u1 (t )IP (t ) − δP IP (t )). (31) Using Pontryagin’s Maximum Principle [47], the derivatives of the Hamiltonian with respect to the state variables, evaluated at the optimal controls, is given by ∂H ∂H ∂H ∂H , λ =− ,λ = − , and λIP = − . ∂C E ∂E I ∂I ∂ IP λC = − (32) Thus adjoint system (26)-(29), with transversality conditions (terminal conditions) expressed in the form provided in (30), was obtained from the Hamiltonian (31) and the relations in (32). The Hamiltonian H is minimised with respect to the controls at the optimal control pair u∗ = (u∗1 , u∗1 ). Thus, we differentiate H with respect to u1 and u2 on the interior sets {t | 0 ≤ u1 ≤ m1 } and {t | 0 ≤ u2 ≤ m2 } respectively. Hence, the optimality equations are ∂H = A1 u∗1 + ρ IP∗ λI − γ QI∗ λIP − ρ IP∗ λIP = 0 ∂ u1 ∂H = A2 u∗2 − P k2 I∗ λC = 0 at u∗2 . ∂ u2 at u∗1 , (33) (34) Solving for u∗1 and u∗2 on the interior sets, we obtain u∗1 = u∗2 = γ QI∗ λIP + ρ IP∗ λIP − ρ IP∗ λI P k2 I λC . A2 A1 , (35) ∗ (36) By standard control arguments involving the bounds on the controls (see Sections 8.1 and 12.1 of [49]), we conclude that ⎧ ⎪ 0 ⎪ ⎪ ⎪ ⎪ ⎨ γ QI∗ λIP + ρ IP∗ λIP − ρ IP∗ λI u∗1 = A1 ⎪ ⎪ ⎪ ⎪ ⎪ ⎩ γ QI∗ λIP + ρ IP∗ λIP − ρ IP∗ λI ≤0 A1 γ QI∗ λIP + ρ IP∗ λIP − ρ IP∗ λI if 0 < < m1 , A1 γ QI∗ λIP + ρ IP∗ λIP − ρ IP∗ λI if ≥ m1 A1 if m1 which in compact notation can be characterised as u∗1 (t ) = min max 0, γ QI∗ λIP + ρ IP∗ λIP − ρ IP∗ λI A1 (37) , m1 . (38) Similarly, we conclude that u∗2 = ⎧ ⎪ 0 ⎪ ⎪ ⎪ ⎪ ⎨ ⎪ ⎪ ⎪ ⎪ ⎪ ⎩ P k2 I∗ λC ≤ 0, A2 P k2 I∗ λC if 0 < < m2 A2 P k2 I∗ λC if ≥ m2 A2 if P k2 I∗ λC A2 m2 (39) which in compact notation can also be characterised as u∗2 (t ) = min max 0, This completes the proof. P k2 I∗ λC A2 , m2 . (40) M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 11 Fig. 3. Numerical simulation of the tryptophan supplementation Chlamydia treatment model (19)-(22) showing the time course plot of (a) C(t), concentration of Chlamydia, (b) E(t), concentration of healthy epithelial cells, (c) I(t), concentration of infected epithelial cells, and (d) IP (t), concentration of persistently infected epithelial cells, respectively, with no controls applied. Note that the final time values of each state variable, which are also their calculated steady states, rounded up to the next integer, are C (t f ) = 51, E (t f ) = 110, I (t f ) = 2, IP (t f ) = 36, respectively. 4. Numerical results In this section, we present numerical results of the model system (19)-(22) under different scenarios. Bacterial Clearance Benchmark: It is estimated that the theoretical limit of detection (LOD) of C. trachomatis using a recombinase polymerase amplification (RPA) assay is 10 0 0 to 240 0 cells/mL of urine [65]. An infected epithelial cell produces about 200 to 500 infectious Chlamydia [42]. Thus we assume that the equivalence of the LOD of infected epithelial cells in a millilitre of genital epithelial tissue is about 2 to 12 epithelial cells. Thus, the LOD of infected/persistent epithelial cells is estimated to be within the range 2 × 10−3 − 12 × 10−3 cells/mm3 , that is [0.002 - 0.012] cells/mm3 , while the LOD of infectious Chlamydia is 1 to 2.4 cells/mm3 , that is [1 - 2.4] cells/mm3 . For simplicity, in this study, we assume that the LOD of infected or persistently infected epithelial host cells is 0.01 cells/mm3 , while that of infectious Chlamydia is 1 cell/mm3 . 4.1. Disease dynamics with no treatment (control) In this section, we present numerical results for the dynamics of the extracellular Chlamydia, healthy epithelial cells, infected epithelial cells, and persistently infected epithelial cells. We solve the system of ODEs of the basic model system (1)-(4), which is synonymous to solving the model system (19)-(22), with u1 = u2 = 0. Fig. 3a–d show that in the absence of any therapeutic intervention, the infection may not be abated, even when the antimicrobial responses of the humoral and cell-mediated immunity are present. It is observed that each of the interacting species approached their endemic states. The parameter values and initial conditions used in all the simulations are given in Table 1. 12 M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 4.2. Optimal control In this section, we numerically study and present the numerical solutions of the optimality system described in Section 3.3, over t f = 5 days. The choice of a five day regimen is influenced by the fact that a single dose treatment may not suffice for the clearance of a chronic chlamydial infection as this requires higher doses and a longer duration of antibiotics [28]. In addition, should multiple drug doses be recommended for the achievement of the treatment guidelines the model results will suggest, there may be concerns about patient compliance to multiple drug doses. Hence the choice of five days, which is shorter than the recommended seven day treatment regimen of doxycycline [5,14]. The goal of this study is to find the optimal treatment regimen, that is the most effective and efficient temporal drug usage, that ensures the clearance of a chronic Chlamydia infection, whilst minimizing the drugs levels and their systemic costs. Numerical techniques for optimal control problems can be classified as either direct or indirect (See [69] and [70, Pg. 161]). The initial solutions of the two-point boundary value problem described in Theorem 3.2 were obtained by using an indirect method in which the differential-algebraic system generated by Pontryagin’s Maximum Principle is numerically solved, a numerical technique generally referred to as the Forward-Backward Sweep Method (FBSM) [49,69]. First, using a fourth order Runge-Kutta scheme, the state equations are solved forward in time, with initial conditions (see Table 1) and initial guesses for the controls. By virtue of the transversality conditions (30) being at the final time, the adjoint equations are solved by a backward (in time) fourth order Runge-Kutta scheme, using the current iteration solution of the state equations. The controls are then updated by using a convex combination (ui = 0.5(ui,prev + ui,char )) of the previous controls (ui, prev ) and the value from the characterisations (38) and (40) (ui,char , i = 1, 2). This process is repeated and the iteration stops when convergence is achieved [49]. The described iterative method of obtaining the optimal treatment strategy was used to obtain solutions of the system when either controls was used. However, when both controls were used, the simulations took several hours to run, which made variation of parameters a time-consuming task. Thus, in addition to the method, the optimal control problem (19)-(25) was solved using MATLAB’s in-built non-linear optimisation tool fmincon [71]. fmincon is a constrained optimization toolbox that is based on a direct (sequential) method in which the differential Eqs. (19)-(22) and the integral (25) are discretised, and the problem is converted into a nonlinear programming problem [69,70]. We first reformulate the optimal control problem into the Mayer form (see [72]) and then set it up for fmincon [71]. In order to investigate the occurrence of any discrepancy in the solutions obtained when either the FBSM or MATLAB’s fmincon was used, fmincon was used to regenerate some of the previous results obtained from the FBSM. We note that there were no discrepancies. In order to obtain an optimal therapy during a chronic chlamydial infection, we assume that at the time of commencement of the treatment, the infected host has reached chronic steady state values C = 51 cells/mm3 , E = 110 cells/mm3 , I = 2 cells/mm3 , and IP = 36 cells/mm3 , respectively. These steady state values were obtained by setting the right hand size of the model system (19)-(22) to zero in the absence of any treatment, that is u1 ≡ u2 ≡ 0, and solving the resulting system of nonlinear equations using MATLAB’s in-built solver fsolve. We investigate and compare numerical results of three different optimal therapy scenarios: (i) when u1 , tryptophan supplement, is optimised while treatment u2 is set to zero, (ii) when u2 , the drug that is bacteriostatic on Chlamydia, is optimised while treatment u1 is set to zero, and (iii) when both treatments u1 and u2 are optimised. We only track the amount of bio-available treatment/drug the system is supplied with, with respect to time. In this study, we do not investigate the complete degradation of the drug/treatment to be administered, but rather the bio-availability and delivery of the treatment into the system. We simulated the model for different combination of values of the weight parameters A1 , A2 , A3 , A4 , A5 , and A6 , which are the balancing cost factors due to scales (that is, they adjust the balance between the clearance of the infection and the systemic cost of the treatments) and the importance of the five parts of the objective functional. In all the presented figures (except otherwise stated), we use the same set of weight factors, A1 = 5, A2 = 1, A3 = 15, A4 = 50, and A5 = 30, to illustrate the effects of various optimal therapies on a chlamydial infection. Note that the initial conditions for all the simulations of the sub-models in this section are the steady state solutions of the no-treatment model, that is C (t0 ) = 51, E (t0 ) = 110, I (t0 ) = 2, and IP (t0 ) = 36. The numerical results of different treatment combinations are presented in the sections that follow. 4.2.1. Control with bacteriostatic agents only (u1 ≡ 0) We consider monotherapy with the bacteriostatic agent represented by u2 , and with u1 ≡ 0. Numerical simulations are performed using MATLAB’s fmincon as described in Section 4.2, for A2 ≡ 0 when u1 ≡ 0. Then, the optimal control problem is defined by J ( u2 ) = tf t0 G(t , x(t ), u2 (t ))dt + φ (x(t f )), (41) subject to x˙ = f (t, x(t ), u2 (t )), x(t0 ) = x0 , t0 ≤ t ≤ t f , (42) M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 13 Fig. 4. Treatment 1: Effects of bacteriostatic monotherapy on (a) C(t), concentration of Chlamydia and E(t), concentration of healthy epithelial cells; (b) I(t), concentration of infected epithelial cells, and IP (t), concentration of persistently infected epithelial cells, respectively. (c) Optimal evolution for control u2 . Note that the final time values of the state variables are 0.0081, 154.1020, 0.0004, and 11.2736, respectively. where x = (C, E, I, IP ), f is the right side of the model (state) system (19)-(22), φ (x(t f )) = A3C (t f ) + A4 I (t f ) + A5 IP (t f ), and A G(t, x, u2 ) = 22 u22 . For this therapy, as explained in Section 3.1, μτ = 0. Thus, μ = 2 (see Table 1). As shown in Fig. 4, the optimal control problem predicts the clearance of ‘active’ Chlamydia infection at the cessation of the therapy (C (t f ) = 0.0081 cells/mm3 and I (t f ) = 0.0 0 04 cells/mm3 , both below the LOD) in the presence of bacteriostatic agents alone. It also however shows that the chronic chlamydial infection, which is characterised by the presence of inflammation-inducing persistently infected epithelial cells at the end of therapy (IP (t f ) = 11.2736 cells/mm3 ), may not be abated by bacteriostatic agents alone. The optimal control function u2 (t) for these results, as shown in Fig. 4c, is continuous and decreases with respect to time. The simulation results show that despite the clearance of active intracellular and extracellular Chlamydia, the optimal control model does not result in the total clearance of the chronic infection even when u2 = 0.9 (the maximum tissue concentration of the drug) for about three and a half days, with lesser concentrations till the end of the therapy, as shown in Fig. 4c. Hence, there is the possibility of the sufficient residue of persistent Chlamydia to reactivate from their latent form, thereby enhancing future (auto) reinfection [8,18,19]. Even in the absence of chlamydial reactivation, persistent Chlamydia forms induce inflammatory responses that lead to the scarring of genital tissues and other severe sequelae [24–26]. This is undesirable. Hence, we explore other therapeutic strategies for the absolute clearance of all chlamydial forms at treatment cessation. 4.2.2. Control with tryptophan supplementation only (u2 ≡ 0) We again consider monotherapy, this time with u1 , the tryptophan supplement alone. Numerical simulations are performed using MATLAB’s fmincon as described in Section 4.2, for A2 ≡ 0 when u2 ≡ 0. Then, the optimal control problem is defined by relations similar to (41) and (42), but with u1 instead of u2 , and G = A G(t, x, u1 ) = 21 u21 . For this therapy, tryptophan alone acts by reversing chlamydial persistence since it facilitates the redifferentiation of persistent Chlamydia into normal RBs and infectious EBs [19,73,74]. Thus, μτ = 0 and μ = 2 (see Table 1). As shown in Fig. 5, the optimal control problem predicts, unsurprisingly, that although the severity of the chronic chlamydial infection is reduced, the infection will not be abated by the end of the therapy. This is characterised by the presence of 14 M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 Fig. 5. Treatment 2: Effects of tryptophan monotherapy on (a) C(t), concentration of Chlamydia and E(t), concentration of healthy epithelial cells; (b) I(t), concentration of infected epithelial cells, and IP (t), concentration of persistently infected epithelial cells, respectively. (c) Optimal evolution for control u1 . Note that the final time values of the state variables are 26.6300, 74.4130, 0.6618, and 20.4631, respectively. (detectable) infectious Chlamydia bacteria (26.6300 cells/mm3 ), infected epithelial cells (0.6618 cells/mm3 ) and persistently infected epithelial cells (20.4631 cells/mm3 ). It can also be seen that healthy epithelial cells have not proliferated normally by the end of the therapy (74.4130 cells/mm3 ). The optimal control function u1 (t) in Fig. 5c is continuous and decreases with respect to increasing time. The simulation results show that for the described therapeutic effects to be obtained, the optimal control will be the maximum tissue concentration of the tryptophan supplement for about two days, as shown in Fig. 5c. 4.2.3. Control with tryptophan and bacteriostatic agent only With this treatment strategy, the tryptophan supplementation control u2 , and bacteriostatic control u1 , are both used to optimise the objective functional J, as in (25). We note that the treatment does not include a cocktail of tryptophan and L-1MT, as such, Eq. (22) does not contain the reduction term 1 − u1 . For this therapy, as explained in Section 2, μτ , the rate at which the tryptophan supplement facilitates the reduction in the amount of EBs produced on lysis of infected epithelial cells, is 1 day−1 . As shown in Fig. 6, the optimal control problem predicts that the chronic chlamydial infection will be abated when both tryptophan supplement and bacteriostatic agents are used for the treatment of a chronic genital chlamydial infection. It can be seen that in the presence of the two controls, active Chlamydia and infected cells were cleared (C (t f ) = 0.0422 cell/mm3 and I (t f ) = 0.0048 cells/mm3 ), while healthy epithelial cells recovered from their diminished state and proliferated normally (E (t f ) = 152.0573 cells/mm3 ), and persistently infected cells were significantly cleared (IP (t f ) = 0.0440 cells/mm3 , howbeit with a detectable and inflammation-inducing remnant. This result suggests that the inhibitory properties of tryptophan on the development of persistent Chlamydia may not suffice for clearing a chronic chlamydial infection. The optimal control functions u1 (t) and u2 (t), as shown in Fig. 6c are continuous and decrease with respect to time. Our simulations show that for the described therapeutic effects to be obtained, the optimal control u1 (t) will be about half the maximum tissue concentration of the tryptophan supplement given throughout the treatment period, while the optimal control u2 (t) will be the maximum tissue concentration of the bacteriostatic agent administered for about four and a half days. 15 t t M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 t Fig. 6. Treatment 3: Effects of combination therapy comprising tryptophan supplementation and bacteriostatic agents on (a) C(t), concentration of Chlamydia andE(t), concentration of healthy epithelial cells; (b) I(t), concentration of infected epithelial cells, and IP (t), concentration of persistently infected epithelial cells, respectively. (c) Optimal evolution for both controls u1 and u2 . Note that the final time values of the state variables are 0.0422, 152.0573.0.0048, and 0.0440, respectively. 4.2.4. Optimal combination treatment: Cocktail of tryptophan and L-1MT with bacteriostatic agent We further investigate the addition of L-1MT to the therapy, with the assumption that its immunomodulatory properties would yield an improved prognosis. With this treatment strategy, all the proposed treatments/drugs (trytophan supplement, L-1MT, and bacteriostatic agent) are combined. Thus, controls u1 and u2 are used to optimise the objective functional J as in (25). Consequently, the full model system (19)-(22) is implemented. For this therapy, as explained in Section 2, μτ , the rate at which the (more potent) cocktail of tryptophan supplement and L-1MT facilitates the reduction in the amount of EBs produced on lysis of infected epithelial cells, is 2 day−1 , and ρ = 15. As shown in Fig. 7, the optimal control problem predicts that similar to the results in Section 4.2.3, the chronic chlamydial infection will be cleared when the treatment cocktail is used for the treatment of a chronic genital chlamydial infection. It can be seen that in the presence of the two controls, active Chlamydia was cleared (C (t f ) = 0.0022 cell/mm3 ), and both persistent and non-persistent Chlamydiae were cleared (I (t f ) = 0.0 0 01 cells/mm3 and IP (t f ) = 0.0104 cells/mm3 , respectively), while healthy epithelial cells recovered from their diminished state and proliferated normally (E (t f ) = 153.1859 cells/mm3 ). The significant differences between these results and those of Section 4.2.3 are in the clearance of persistent Chlamydia and in the tissue concentrations of treatments/drugs required to achieve this. While the optimal control functions u1 (t) and u2 (t), as shown in Fig. 7c are continuous and decrease with respect to time like the previous results, simulation results show that for the described therapeutic effects to be obtained, the optimal controls u1 (t) and u2 (t) will first be given as an initial pulse of about half their maximum tissue concentrations on the first day of treatment. This will be followed by a higher tissue concentration of u1 (t), and the maximum tissue concentration of u2 (t) at about 0.2 days ( ≈ 5 hours) post-treatment initiation. 4.2.5. Varying the weights on controls u1 (t) and u2 (t), and interacting species C(t), I(t), and IP (t) In this section, building on the optimal control strategy described in Section 4.2.4, we further investigate the effects of varying weight parameters A1 and A2 , the balancing cost of implementing the respective treatments, on the optimal evolution for both controls u1 (t) and u2 (t), respectively, and the weights A3 , A4 , and A5 , on the interacting species C(t), I(t), and IP (t), respectively. 16 M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 Fig. 7. Treatment 3: Effects of combination therapy comprising cocktail of tryptophan supplement and L-1MT, and bacteriostatic agents, on (a) C(t), concentration of Chlamydia and E(t), concentration of healthy epithelial cells; (b) I(t), concentration of infected epithelial cells, and IP (t), concentration of persistently infected epithelial cells, respectively. (c) Optimal evolution for controls u1 and u2 . Note that the final time values of the state variables are 0.0022, 153.1859, 0.0001, and 0.0104, respectively. We vary the weight A1 , which represents the cost associated with administering the tryptophan cocktail, using weights A1 = 5, A1 = 50, A1 = 500, and A1 = 10 0 0. These various weights correspond to higher systemic costs of the tryptophan cocktail to the host as the weight increases. The optimal evolution for both controls u1 (t) and u2 (t) are shown on Fig. 8c and d, respectively. Fig. 8 shows that as the weight parameter A1 increases, there was a slight decline in the initial concentrations of Chlamydia and infected epithelial cells. There was however an increase in the concentrations of persistently infected cells, which persisted at the cessation of the therapy for higher weight values (50 - 10 0 0). The objective functional J(u1 (t), u2 (t)) (25) also increased significantly with increasing A1 values (see Table 2). Fig. 8c however shows that as A1 increases, control u1 (t) decreased in level, howbeit with a longer duration of about four and a half days, while Fig. 8d shows that control u2 (t) increased in duration, and was at the maximum level throughout the therapy. Despite these adjustment in the tissue concentration of the treatments, persistently infected cells were still detectable (for A1 = 50 0, 10 0 0) as depicted by the final concentrations of the interacting species at the end of treatment, as shown in Table 2. These results suggest that lower systemic costs of the tryptophan cocktail are required to achieve shorter treatment durations and a total clearance of the chronic chlamydial infection by the cessation of therapy. We vary the weight A2 , which represents the cost associated with administering the bacteriostatic agent, using weights A2 = 1, A2 = 10, A2 = 100, and A2 = 10 0 0. These various weights correspond to higher systemic cost of the bacteriostatic agent to the host as the weight increases. The optimal evolution for both controls u1 (t) and u2 (t) are shown on Fig. 9c and d. Fig. 9 shows that as the weight parameter increased from A2 = 1 to A2 = 10, there was an initially significant exacerbation of the infection, characterised by a higher bacteraemia and a deeper plunge in the concentration of healthy epithelial cells. However, as A2 increased further, there were insignificant changes in concentrations of the interacting species. Fig. 9c however shows that as A2 increased, control u1 (t) increased in level and duration, while Fig. 9d shows that control u2 (t) decreased in level. Despite these adjustment in the tissue concentration of the treatments, persistently infected cells were still detectable (for A2 = 10, 100), and the concentration of healthy epithelial cells declined, as depicted by the final concentrations of the interacting species at the end of treatment, as shown in Table 2. These results suggest that a low systemic costs of the bacteriostatic agent, when combined with the tryptophan cocktail, suffices for the achievement of a total clearance of the chronic chlamydial infection by the cessation of therapy. M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 17 Fig. 8. Effects of varying weight A1 : Numerical simulation of the Tryptophan supplementation Chlamydia treatment model (19)-(22) showing the time course plot of (a) C(t), concentration of Chlamydia, and E(t), concentration of healthy epithelial cells; (b) I(t), concentration of infected epithelial cells, and IP (t), concentration of persistently infected epithelial cells; (c) Solution profile of control u1 (t), the tryptophan cocktail; and (d) Solution profile of control u2 (t), the bacteriostatic agent, respectively. We vary the weight A3 , systemic cost of clearing Chlamydia using weights A3 = 15, A3 = 150, A3 = 500, and A3 = 10 0 0. The effect of this variation on interacting species was insignificant as shown on Fig. 10. Fig. 10c however shows that as A3 increased, slightly higher levels, with slightly shorter durations, of control u1 (t), were required to achieve the described therapeutic effects, while Fig. 10d show that higher levels of control u2 (t) were required towards the end of the therapy (for about one and a half days before cessation of treatment). The objective functional also increased insignificantly as shown on Table 2. These adjustments in the tissue concentration of the treatments ensures that the chronic chlamydial infection is totally cleared as depicted on Fig. 10 and Table 2. We vary the weights A4 , systemic cost of clearing the Chlamydia-infected cells I(t) using weights A4 = 50, A4 = 200, A4 = 500, and A4 = 1000. The effect of this variation on interacting species was insignificant. The time courses of the interacting species are similar to those observed in Fig. 7, as shown on Fig. 11. Fig. 11c and d depict the optimal evolution for controls u1 (t) and u2 (t). As A4 increased, insignificantly higher levels, with insignificantly shorter durations, of control u1 (t), were required to achieve the described therapeutic effects while slightly higher levels of control u2 (t) were required towards the end of the therapy (for about one and a half days before cessation of treatment). The objective functional also increased insignificantly as shown on Table 2. These adjustments in the tissue concentration of the treatments ensures that the chronic chlamydial infection is totally cleared as depicted on Fig. 11 and Table 2. We vary the weights A5 , systemic cost of clearing the persistently-infected epithelial cells IP (t) using weights A5 = 30, A5 = 200, A5 = 500, and A5 = 10 0 0. These various weights correspond to higher host systemic cost as the weight increases. Fig. 12 shows that as the weight parameter increased from A5 = 30 to A5 = 200, there was an initially significant exacerbation of the infection, characterised by a higher bacteraemia and a deeper plunge in the concentration of healthy epithelial cells (REF FIG). However, as A5 increased further, there were insignificant changes in concentrations of the interacting 18 M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 Table 2 Summary of variations in the weight parameters and their corresponding effects. The subscript ’end’ indicates the listed values of each variable at the cessation of the therapy, that is when t = t f . A1 A2 A3 A4 A5 Cend Eend Iend IPend Jend u1end u2end 5 50 500 1000 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 5 1 1 1 1 1 10 100 1000 1 1 1 1 1 1 1 1 1 1 1 1 15 15 15 15 15 15 15 15 15 150 500 1000 15 15 15 15 15 15 15 15 50 50 50 50 50 50 50 50 50 50 50 50 50 200 500 1000 50 50 50 50 30 30 30 30 30 30 30 30 30 30 30 30 30 30 30 30 30 200 500 1000 0.0022 0.0140 0.0588 0.0846 0.0022 0.0465 0.2017 0.3760 0.0022 0.0006 0.0003 0.0001 0.0022 0.0019 0.0014 0.0011 0.0022 0.0058 0.0064 0.0061 153.1859 153.9023 153.8840 153.8578 153.1859 135.0756 135.4848 135.6262 153.1859 153.8242 154.0426 154.1042 153.1859 153.3335 153.5153 153.6761 153.1859 141.1912 140.8363 140.9951 0.0001 0.0023 0.0098 0.0141 0.0001 0.0024 0.0102 0.0197 0.0001 0.0000 0.0000 0.0000 0.0001 0.0001 0.0001 0.0001 0.0001 0.0019 0.0018 0.0015 0.0104 0.0436 0.3107 0.5415 0.0104 0.0316 0.0327 0.0015 0.0104 0.0095 0.0092 0.0090 0.0104 0.0102 0.0100 0.0099 0.0104 0.0010 0.0004 0.0002 2.5164 6.7993 23.0732 32.9086 2.5164 7.0750 9.9661 12.6867 2.5164 2.6271 2.7370 2.8179 2.5164 2.5309 2.5505 2.5732 2.5164 2.8926 3.0170 3.1374 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.6844 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.6774 0.9000 0.9000 0.1190 0.9000 0.9000 0.9000 0.1190 0.2267 0.0938 0.0180 0.1190 0.3582 0.5182 0.6158 0.1190 0.1121 0.1055 0.1017 0.1190 0.9000 0.9000 0.8998 Fig. 9. Effects of varying weight A2 : Numerical simulation of the Tryptophan supplementation Chlamydia treatment model (19)-(22) showing the time course plot of (a) C(t), concentration of Chlamydia, and E(t), concentration of healthy epithelial cells; (b) I(t), concentration of infected epithelial cells, and IP (t), concentration of persistently infected epithelial cells; (c) Solution profile of control u1 (t), the tryptophan cocktail; and (d) Solution profile of control u2 (t), the bacteriostatic agent, respectively. M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 19 species. Fig. 12c however shows that as the weight increased from A5 = 30 to A5 = 200, lower levels of control u1 (t) were initially required at the beginning of the therapy. This however increased exponentially, up to the maximum tissue concentration, at the cessation of therapy. On the other hand, control u2 (t) was also required at lower levels at the beginning of the therapy, but a high tissue concentration is to be maintained till the cessation of the therapy, as shown on Fig. 12d. However, as A5 increased further, there were insignificant changes in the levels of both controls. These adjustments in the tissue concentration of the treatments ensures that the chronic chlamydial infection is totally cleared as depicted on Fig. 12 and Table 2. Summarily, this investigation shows that low systemic costs of the different arms of the treatment strategy are required and suffice for the clearance of a chronic chlamydial (genital) infection. 5. Discussion and conclusion Chlamydia trachomatis is the most commonly reported sexually transmitted bacterial disease of the developed world [7,14,28,75,76]. Its ocular serovar is responsible for the world’s leading cause of preventable blindness, can be treated with antibiotics [28,76,77]. In the advent of more sensitive diagnostic Chlamydia assays, treatment failures have been recorded, many of which are consequences of chronic Chlamydia infections characterised by aberrant and persistent chlamydial forms, which are resistant to antibiotic activity, and are the major cause of the severe sequelae of C. trachomatis infection [14,28,75]. Thus, there is a need for the international treatment guideline of Chlamydia to be re-evaluated [28,75]. In this study, we investigate different treatment strategies for the treatment of a chronic genital chlamydial infection characterised by interferon-gamma-induced chlamydial persistence. This investigation is motivated by recent studies of the reactivation of latent pathogens [32,35,36], such as Chlamydia [38–41], which would then allow for further clearance by bacteriostatic/bactericidal agents and the immune system. A number of studies have shown that tryptophan is able to reverse chlamydial persistence, while the tryptophan derived from Levo-1-methyl tryptophan (L-1MT) is able to both reverse and inhibit IFN-γ -induced chlamydial persistence [28–30] (while also facilitating a reduction in the burst size of lysing infected cells), thereby making them more sensitive to antibiotic (or bacteriostatic) activity, which acts only on metabolically active Chlamydia forms [28]. It has also been suggested that chronic chlamydial infection require higher doses and duration of antibiotics [28]. Thus, we hypothesised that a combination of these treatments (tryptophan, L-1MT, and bacteriostatic agents) will efficiently reverse IFN-γ -induced chlamydial persistence, and ultimately clear a chronic Chlamydia infection [28]. We used an optimal control theory paradigm to model the treatment of chronic chlamydial infection. Our approach couples an existing model of within-host interaction of Chlamydia and the immune system [42], with an additional class for persistently infected epithelial cells. We have investigated the dynamics of the interacting species with and without two different therapeutic controls/treatments. Mathematical analysis of the base model shows that the disease-free equilibrium is globally asymptotically stable whenever the associated basic reproduction number R0 is less than unity and unstable otherwise. Using the Latin Hypercube Sampling (LHS) and partial rank correlation coefficient (PRCCs) schemes, uncertainty and global sensitivity analyses were performed in order to determine the model parameters that are most influential on the model’s R0 . Sensitivity analysis results suggest that an effective treatment/control strategy for chronic chlamydial infections should reduce a combination of one or more of ((i) P, the burst size per infected cell, (ii) k1 , the rate of cell infection, (iii) k2 , the rate of lysis of infected cells, and (iv) PE , the production rate of mucosal epithelial cells. Obviously, it may not be desirable to perturb PE . The results also suggest that an improvement on the host system’s ability to clear the infection (via the effectiveness of the humoral (μ) and cell mediated (γ ) immunities), or the rapid death of epithelial cells (δ E ), though undesirable, will abate the chlamydial infection. Taking these results into account, and utilising the reactivation and inhibitory properties of tryptophan derivatives on chlamydial persistence, we use methods of optimal control to derive and analyse the conditions for optimal treatment of the disease using a combination of a tryptophan supplement, L-1MT, and a bacteriostatic agent/drug. Existence and uniqueness of the optimal controls were proved. We also characterised the controls using Pontryagin’s Maximum Principle and the resulting optimality system was numerically solved. Our optimal control problem accounts for: (i) the blockage of IFNγ -induced persistence by tryptophan supplementation and L-1MT; (ii) the reversal of established chlamydial persistence induced by IFN-γ , using L-1MT; (iii) the effects of the humoral and cell-mediated immune responses; (iv) the antimicrobial effects of an antibiotic therapy; (v) reduction of the production of EBs; and (vi) a five day duration of treatment with minimal dosage administration. We numerically explored the different impacts of the two optimal controls under four different treatment combinations. The optimal control problem indicates the necessity of the high immunomodulatory effects of tryptophan supplementation and L-1MT. The numerical results of the model suggest that combination therapy with a cocktail of tryptophan supplement, L-1MT, and a bacteriostatic agent, may be the optimal strategy for the treatment of a chronic Chlamydia infection, as it aids the clearance of the pathogen itself, clearance of chlamydial persistence, limitation of side effects of drugs, and the survival of healthy epithelial cells. The optimal therapy is an initial pulse of about half the maximum (bioavailable) tissue concentration of both the bacteriostatic agent and the cocktail of tryptophan and L-1MT. At about 0.2 days ( ≈ 5 hours) post-treatment initiation, this should be followed by a concurrent treatment with the cocktail of tryptophan and L-1MT (at the maximum bioavailable tissue concentration) and the bacteriostatic agent (at about half the maximum bioavailable tissue concentration) until about day three post-treatment initiation. Our study also showed that lower weights on the controls, which correspond to lower systemic costs of treatments to the host, are more effective and suffice for the clearance of a 20 M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 chronic chlamydial (genital) infection. We suggest that therapeutic interventions that are inclined to this treatment guideline may be effective in treating chronic Chlamydia infections [14,28]. We importantly acknowledge the likely adverse effect of tryptophan on the host immune system. When a tryptophan supplement is taken, indoleamine 2,3-dioxygenase (IDO) 1 is expressed. This leads to tryptophan depletion and the generation of bioactive catabolites known as kynurenines. This process can induce the suppression of the innate and adaptive immunity by some cells of the immune system, thereby promoting tolerogenic responses. The kynurenine pathway of tryptophan catabolism modifies immunological and neurological responses to inflammation. It promotes neurological comorbidities such as pain, depression, and fatigue [78]. These are undesirable health consequences. As such, the use of tryptophan supplements in the treatment of chronic chlamydial infection should be used with caution and subjected to thorough clinical tests. The optimal treatment regimen suggested by our model require that strong doses of the drug cocktail - bacteriostatic agents, L-1MT, and tryptophan supplements - be administered and maintained within a therapeutic band for days. This could mean that patients have to take multiple doses of the drugs. This may not be plausible because of the issue of patients’ compliance to multiple drug dosages [14,79]. However drug delivery systems that can make such treatment regimens a reality can be designed. Effective drug delivery systems, that can ensure a controlled release/delivery of drugs, while also maintaining the drugs’ (tissue/plasma) concentration within a therapeutic band, over a particular period of time, have previously been engineered. One such application is the use of polymer therapeutics, a system that can use polymer chain as the inert carrier to which a drug is covalently linked. Such conjugation can be used to elicit in vivo spatiotemporal release of drugs designed to attain desired therapeutically effective concentration, with other benefits including reduction in immunogenicity, protection of the drug from proteolytic enzymes, potential for targeted delivery, and increased plasma halflife, which would imply that less frequent doses of the drug are required [80,81]. Diseases for which polymer conjugates have been successfully designed include hepatitis B and C, and ischemia [81,82]. Such new and bio-compatible drug delivery system that can enhance the permeability and spatiotemporal release of chlamydial antibiotics (such as azithromycin, which already has a long half-life) at concentrations higher than the minimum inhibitory concentration (MIC) of Chlamydia [75], may facilitate its bioavailability at very high concentrations for several days. The use of a transdermal patch to constantly deliver desired concentrations of the drug over time may also be able to provide the therapeutically effective drug concentration that suits our model’s suggested optimal treatment strategy/regimen. The fact that model parameters that describe biological processes may have been over-estimated is a limitation of this study. In particular, the effects of the cell-mediated immune response may have been over-emphasised, thereby resulting in an improved clearance of a chlamydial infection as compared to what happens in vivo. The presented model has been kept fairly generic and further studies are required in order for a more accurate model of the interaction between Chlamydia, host epithelial cells, and the immune response, to be incorporated. In addition, direct pharmacology for monotherapy and combination therapy of treatments need to be investigated further. Our optimal control model represents a general framework for analysing the potential clinical effectiveness of novel combination regimens directed at a wide range of infectious diseases particularly those involving latent forms, such as Chlamydia, HIV and tuberculosis. In the case of Chlamydia trachomatis, particularly, combination therapies could be studied in the context of more detailed models for persistence induction. While our present study focusses exclusively on IFN-γ -induced chlamydial persistence, several other persistence-inducing mechanisms have been identified which could be explored in future using a similar modelling framework to the one we present here. These additional persistence-inducing mechanisms include antibiotic-induced persistence (as observed for number of classes of antibiotics such as penicillin, ofloxacin, and ciprofloxacin), deficiency-induced persistence due to the depletion of essential nutrients, and persistence induced by monocyte infections, phage infection of Chlamydiae, and continuous infection [17]. The gastrointestinal tract has also been implicated in the development of persistent infection with Chlamydia, due to autoinoculation of the genital tract by Chlamydia organisms shed in the rectum [83–85]. Appendix A. Basic properties of model without treatment A.1. Positivity of solutions The model system (1)-(4) describes the dynamics of cell populations. Hence, it is essential that all its state variables remain non-negative for all time. This implies that the solutions of the system will remain positive for all t > 0 when given positive initial conditions. We establish this important condition via the following lemma: Lemma A.1. Given non-negative initial values of the state variables in Eqs. (1)-(4), non-negative solutions are generated for all time t > 0. Proof. Let (C(0), E(0), I(0), IP (0)) be a positive initial condition and denote by [0, tmax ], the maximum interval of existence of the corresponding solution. In order to prove that the solution is positive in [0, +∞], it suffices to show that it is positive in [0, tmax ]. Let ts = sup{0 < t < tmax : C (t ) > 0, E (t ) > 0, I (t ) > 0, IP (t ) > 0 on [0, t]}. Thus, ts > 0, since C(0), E(0), I(0), and IP (0) are non-negative. Suppose ts < tmax . From Eq. (1), dC + (μ + k1 E )C = P k2 I. dt M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 Thus, t t μt + k1 E (τ )dτ = P k2 I (t ) exp μt + k1 E ( τ ) dτ , d C (t ) exp dt so that C (ts ) exp Hence, 21 0 0 ts ts τˆ μts + k1 E (τ )dτ − C (0 ) = P k2 I (τˆ ) exp μτˆ + k1 E (τ )dτ dτˆ . 0 0 0 ts ts μts + k1 E (τ )dτ + exp − μts + k1 E (τ )dτ 0 0 ts τˆ × P k2 I (τˆ ) exp μτˆ + k1 E (τ )dτ dτˆ C (ts ) = C (0 ) exp − 0 0 > 0. It can be shown by a similar argument that E(ts ) > 0, I(ts ) > 0, and IP (ts ) > 0. This contradicts the fact that ts is the supremum because at least one of the state variables should be equal to zero at ts . Therefore ts = tmax . Thus, C(t) ≥ 0, E(t) ≥ 0, I(t) ≥ 0, and IP (t) ≥ 0, for all time t > 0. This completes the proof. A.2. Invariant region We consider the long term behaviour of the system (1)-(4) in an apposite biologically feasible region. Consider the region D = (C (t ), E (t ), I (t ), IP (t )) ∈ R4+ : C (t ) ≤ n¯1 , E (t ) ≤ E ∗ , I (t ) ≤ n¯2 , IP (t ) = n¯3 , with n¯1 = e −μt C ( 0 ) + P k2 t 0 t I (τ )eμτ dτ , n¯2 = e−k2 I (0 ) + k1 t 0 (43) t C (τ )E (τ )ek2 τ dτ , and n¯3 = e−δP IP (0 ) + γ Q t 0 I ( τ ) e δ P τ dτ . Lemma A.2. The biologically feasible region D is positively invariant and attracting with respect to the model system (1)-(4) with initial conditions in R4+ . Proof. Since all the parameters and state variables of model system (1)-(4) are non-negative for all t ≥ 0, from the Eq. (1), it follows that dC = P k2 I − μC − k1CE dt ≤ P k2 I − μC. This implies that dC + μC ≤ P k2 I. dt Thus, C (t ) ≤ C (0 )e−μt + e−μt P k2 t 0 I (τ )eμτ dτ . Since the interval [0, t] is compact, and since the integrand, I(τ )eμτ , is continuous on that interval, the corresponding t integral 0 I (τ )eμτ dτ is finite. Therefore C (t ) ≤ e −μt C ( 0 ) + P k2 t 0 I (τ )eμτ dτ From Eq. (2), dE ≤ PE − δE E. dt This implies that dE + δE E ≤ PE . dt Thus, E (t ) ≤ E (0 )e−δE t + PE δE (1 − e−δE t ), = E (0 )e−δE t + E ∗ (1 − e−δE t ), = n¯1 . 22 M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 = E ∗ − (E ∗ − E (0 ))e−δE t . E(t) either approaches E∗ asymptotically or there exists some finite time after which E(t) ≤ E∗ . From Eq. (3), dI ≤ k1CE − k2 I, dt that is dI + k2 I ≤ k1CE. dt Thus, I (t ) ≤ I (0 )e−k2 t + e−k2 t t 0 k 1 C ( τ ) E ( τ ) e k 2 τ dτ . Again, the integral is finite since continuous functions, C (τ )E (τ )ek2 τ is integrated over a compact interval. Therefore I (t ) ≤ e −k2 t I ( 0 ) + k1 t 0 C ( τ )E ( τ )e k2 τ dτ = n¯2 . From Eq. (4), dIP = γ QI − δP IP , dt that is dIP + δP IP = γ QI. dt Thus, IP (t ) = IP (0 )e−δP t + γ Qe−δP t 0 t I (τ )eδP τ dτ . Again, the integral is finite since the continuous functions, I (τ )eδP τ is integrated over a compact interval. Therefore IP (t ) = e−δP t IP (0 ) + γ Q t 0 I (τ )eδP τ dτ = n¯3 . Hence, the region D = (C (t ), E (t ), I (t ), IP (t )) ∈ R4+ : C (t ) ≤ n¯1 , E (t ) ≤ E ∗ , I (t ) ≤ n¯2 , IP (t ) = n¯3 is positively invariant and attracting for the model system (1)-(4), that is, every feasible solution of the model with initial conditions in D, will remain in D, for all t ≥ 0. A.3. Global stability of the Chlamydia-free equilibrium Theorem A.3. The Chlamydia-free equilibrium (CFE) F0 , of the basic Chlamydia persistence model (1)-(4), is globally asymptotically stable in D, whenever R0 < 1 and unstable otherwise. The CFE F0 is the only equilibrium when R0 ≤ 1. Proof. Consider the candidate Lyapunov function Y = P k1 k2 I + k1 (γ + k2 )C, (44) with Lyapunov derivative (where a dot represents differentiation with respect to t) given by Y˙ = P k1 k2 I˙ + k1 (γ + k2 )C˙ = P k1 k2 (k1CE − k2 I − γ I ) + k1 (γ + k2 )(P k2 I − μC − k1CE ) = P k21 k2CE − (k2 (μ + k1 E ) + γ (μ + k1 E ))k1C P k1 k2 E = k1 (k2 + γ )(μ + k1 E ) −1 C (k2 + γ )(μ + k1 E ) ≤ k1 (k2 + γ )(μ + k1 E ∗ ) P k1 k2 E ∗ −1 C (k2 + γ )(μ + k1 E ∗ ) = k1 (k2 + γ )(μ + k1 E ∗ )(R0 − 1 )C ≤ 0, (since E (t ) ≤ E ∗ in D ) when R0 ≤ 1. Since all the model parameters and variables are non-negative, it follows that Y˙ ≤ 0 for R0 ≤ 1, with equality if R0 = 1 or C = 0. Moreover, for R0 < 1, Y˙ = 0 if and only if C = 0. Hence, Y is a Lyapunov function on D . Furthermore, D is a compact M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 23 and absorbing subset of R4+ , and the largest compact invariant set in {(C, E, I, IP ) ∈ D : Y˙ = 0}, when R0 ≤ 1, is the singleton F0 . Therefore, F0 is the only steady state when R0 ≤ 1. Thus, by LaSalle’s invariance principle [86,87], C → 0, I → 0, and IP → 0 as t → ∞. Substituting C = I = IP = 0 into the model Eqs. (1)-(4) shows that E → E∗ as t → ∞. Hence, every solution of the basic Chlamydia model system (1)-(4), with initial conditions in D, approaches the CFE F0 as t → ∞ (that is, the CFE F0 is GAS in D) whenever R0 < 1 and unstable otherwise. A.4. Existence of the Chlamydia-present equilibrium We show that the basic Chlamydia persistence model system (1)-(4) has a unique Chlamydia-present equilibrium (CPE), that is the equilibrium for which Chlamydia persists within-host, if and only if R0 > 1. In order to obtain the CPE, we set the right hand sides of the model Eqs. (1)-(4) to zero, and solve for all its non-zero state variables. We also express the state variables in terms of the force of infection ∗∗ = k1C ∗∗ , (45) of model system (1)-(4). Thus, the right hand sides of the model system (1)-(4) at steady states gives C ∗∗ = ∗∗ PE (Pk2 − k2 − γ ) , μ(δE + ∗∗ )(k2 + γ ) PE E ∗∗ = (46) , (47) δE + ∗∗ ∗∗ PE I∗∗ = , (δE + ∗∗ )(k2 + γ ) γ QI∗∗ IP∗∗ = . δP (48) (49) Thus, the CPE of the basic Chlamydia persistence model (1)-(4) is given by F1 = (C ∗∗ , E ∗∗ , I∗∗ , IP∗∗ ). (50) ∗∗ Substituting (46)-(49) into the expression for in (45), and simplifying, we obtain a quadratic expression that the non-zero CPE F1 of model system (1)-(4) satisfies, that is ∗∗ (μ(k2 + γ )∗∗ + δE μ(k2 + γ ) − k1 PE (Pk2 − k2 − γ )) = 0. (51) The solutions of Eq. (51) are either ∗∗ = 0 (52) or ∗∗ = (k2 + γ )(μ + k1 E ∗ )(R0 − 1 ) . μ/δE (k2 + γ ) (53) The trivial solution (52) implies the disease-free steady state which corresponds to the CFE described by (6). This is not our equilibrium of interest at this point. Thus, the unique and non-trivial solution (53) is valid. From (53), it follows that if R0 < 1, then ∗∗ < 1, which is biologically meaningless. In addition, if R0 = 1, then ∗∗ = 0, which again corresponds to the CFE described by (6). Thus, the model system (1)-(4) has no positive CPE in these two cases. It can be clearly seen that the unique solution (53) of (51) is positive if and only if R0 > 1, since all the model parameters are positive. Hence, the four components C∗∗ , E∗∗ , I∗∗ , and IP∗∗ of the CPE F1 , can be explicitly determined by substituting (53) into (46)-(49), to obtain C ∗∗ = E ∗∗ = PE (k2 (P − 1 ) − γ ) δE − , μ ( k2 + γ ) k1 (54) μ ( k2 + γ ) , k1 ( k2 ( P − 1 ) − γ ) (55) I∗∗ = PE μδE − , k2 + γ k1 ( k2 ( P − 1 ) − γ ) IP∗∗ = γQ PE μδE − . δP k2 + γ k1 (k2 (P − 1 ) − γ ) (56) (57) These results are summarised below. Theorem A.4. The basic Chlamydia persistence model (1)-(4) has a unique CPE given by F1 , whenever R0 > 1 and no CPE otherwise. 24 M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 Appendix B. Figures: Varying the weights on interacting species C(t), I(t), and IP (t) Fig. 10. Effects of varying weight A3 : Numerical simulation of the Tryptophan supplementation Chlamydia treatment model (19)-(22) showing the time course plot of (a) C(t), concentration of Chlamydia, and E(t), concentration of healthy epithelial cells; (b) I(t), concentration of infected epithelial cells, and IP (t), concentration of persistently infected epithelial cells; (c) Solution profile of control u1 (t), the tryptophan cocktail; and (d) Solution profile of control u2 (t), the bacteriostatic agent, respectively. M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 25 Fig. 11. Effects of varying weight A4 : Numerical simulation of the Tryptophan supplementation Chlamydia treatment model (19)-(22) showing the time course plot of (a) C(t), concentration of Chlamydia, and E(t), concentration of healthy epithelial cells; (b) I(t), concentration of infected epithelial cells, and IP (t), concentration of persistently infected epithelial cells; (c) Solution profile of control u1 (t), the tryptophan cocktail; and (d) Solution profile of control u2 (t), the bacteriostatic agent, respectively. 26 M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 Fig. 12. Effects of varying weight A5 : Numerical simulation of the Tryptophan supplementation Chlamydia treatment model (19)-(22) showing the time course plot of (a) C(t), concentration of Chlamydia, and E(t), concentration of healthy epithelial cells; (b) I(t), concentration of infected epithelial cells, and IP (t), concentration of persistently infected epithelial cells; (c) Solution profile of control u1 (t), the tryptophan cocktail; and (d) Solution profile of control u2 (t), the bacteriostatic agent, respectively. M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 27 References [1] C. Kaushic, A.D. Murdin, B.J. Underdown, C.R. Wira, Chlamydia trachomatis infection in the female reproductive tract of the rat: Influence of progesterone on infectivity and immune response, Infect. Immun. 66(3) (1998) 893–898. [2] R.J. Bastidas, C.A. Elwell, J.N. Engel, R.H. Valdivia, Chlamydial intracellular survival strategies, Cold Spring Harb. Perspect. Med. (2013), doi:10.1101/ cshperspect.a010256. [3] R. Rank, J. Whittum-Hudson, Protective immunity to chlamydial genital infection: evidence from animal studies, J. Infect. Dis. 201(S2) (2010) S168–S177. [4] WHO, World Health Organisation: global incidence and prevalence of selected curable sexually transmitted infections 2008, Technical Report, WHO, 2012. Available at: http://www.who.int/hiv/pub/sti/who_hiv_aids_2001.02.pdf. [Accessed January 22, 2014]. [5] F.Y.S. Kong, S.N. Tabrizi, M. Law, L.A. Vodstrcil, M. Chen, C.K. Fairley, R. Guy, C. Bradshaw, J.S. Hocking, Azithromycin versus doxycycline for the treatment of genital Chlamydia infection: a meta-analysis of randomized controlled trials, Clin. Infect. Dis. 59 (2) (2014) 193–205, doi:10.1093/cid/ciu220. [6] R.E. Dixon, S.J. Hwang, G.W. Hennig, K.H. Ramsey, J.H. Schripsema, K.M. Sanders, S.M. Ward, Chlamydia infection causes loss of pacemaker cells and inhibits oocyte transport in the mouse oviduct, Biol. Reprod. 80 (2009) 665–673, doi:10.1095/biolreprod.108.073833. [7] WHO, World Health Organisation: report on global sexually transmitted infection surveillance 2013, Technical Report, Geneva: World Health Organisation, 2014. Available at: http://apps.who.int/iris/bitstream/10665/112922/1/9789241507400_eng.pdf. [Accessed January 22, 2014]. [8] S. Gottlieb, D. Martin, F. Xu, G. Byrne, R. Brunham, Summary: the natural history and immunobiology of Chlamydia trachomatis genital infection and implications for Chlamydia control, J. Infect. Dis. 201(S2) (2010) S190–S204. [9] D.J. Fisher, R.E. Fernandez, N.E. Adams, A.T. Maurelli, Uptake of biotin by Chlamydia spp. through the use of a bacterial transporter (bioy) and a host-cell transporter (SMVT), PLoS ONE 7(9) (2012), doi:10.1371/journal.pone.0046052. 46052 [10] A. Hoare, P. Bavoil, D. Wilson, P. Timms, Spatial constraints within the chlamydial host cell inclusion predict interrupted development and persistence, BMC Microbiol. 8 (1) (2008) 5. [11] R.J.B. Yasser M. AbdelRahman, The chlamydial developmental cycle, FEMS Microbiol. Rev. 29 (2005) 949–959, doi:10.1016/j.femsre.20 05.03.0 02. [12] E.I. Shaw, C.A. Dooley, E.R. Fischer, M.A. Scidmore, K.A. Fields, T. Hackstadt, Three temporal classes of gene expression during the Chlamydia trachomatis developmental cycle, Mol. Microbiol. 37 (4) (20 0 0) 913–925. [13] F. Kong, J. Hocking, Treatment challenges for urogenital and anorectal Chlamydia trachomatis, BMC Infect. Dis. 15 (1) (2015) 293. [14] T.C. Quinn, C.A. Gaydos, Treatment for Chlamydia infection - doxycycline versus azithromycin, N. Engl. J. Med. 373 (26) (2015) 2573–2575 , PMID: 26699174, doi:10.1056/NEJMe1513001. [15] M.A. Kohanski, D.J. Dwyer, J.J. Collins, How antibiotics kill bacteria: from targets to networks, Nat. Rev. Microbiol. 8 (6) (2010) 423–435. [16] O. Peuchant, J.P. Duvert, M. Clerc, S. Raherison, C. Bébéar, C.M. Bebear, B. De Barbeyrac, Effects of antibiotics on Chlamydia trachomatis viability as determined by real-time quantitative PCR, J. Med. Microbiol. 60 (4) (2011) 508–514. [17] R.J. Hogan, S.A. Mathews, S. Mukhopadhyay, J.T. Summersgill, P. Timms, Chlamydial persistence: beyond the biphasic paradigm, Infect. Immun. 72 (4) (2004) 1843–1855, doi:10.1128/IAI.72.4.1843-1855.2004. [18] R.C. Brunham, J. Rey-Ladino, Immunology of Chlamydia infection: Implications for a Chlamydia trachomatis vaccine, Nat. Rev. Immunol. 5 (2005) 149– 161, doi:10.1038/nri1551. [19] R.P. Morrison, New insights into a persistent problemchlamydial infections, J. Clin. Invest. 111 (11) (2003) 1647–1649. [20] M.E. Panzetta, R.H. Valdivia, H.A. Saka, Chlamydia persistence: a survival strategy to evade antimicrobial effects in-vitro and in-vivo, Front. Microbiol. 9 (2018) 3101, doi:10.3389/fmicb.2018.03101. [21] P.E. Munday, B.J. Thomas, C.B. Gilroy, C. Gilchrist, D. Taylor-Robinson, Infrequent detection of Chlamydia trachomatis in a longitudinal study of women with treated cervical infection, Genitourinary Med. 71 (1) (1995) 24–26. [22] U. Dreses-Werringloe, I. Padubrin, H. Zeidler, L. Köhler, Effects of azithromycin and rifampin on Chlamydia trachomatis infection in vitro, Antimicrob. Agents Chemother. 45 (11) (2011) 30 01–30 08. [23] P. Horner, The case for further treatment studies of uncomplicated genital Chlamydia trachomatis infection, Sex. Trans. Infect. 82 (4) (2006) 340–343. [24] M. Malhotra, S. Sood, A. Mukherjee, S. Muralidhar, M. Bala, et al., Genital Chlamydia trachomatis: an update, Indian J. Med. Res. 138 (3) (2013) 303. [25] D. Dean, R.J. Suchland, W.E. Stamm, Evidence for long-term cervical persistence of Chlamydia trachomatis by Omp1 genotyping, J. Infect. Dis. 182 (20 0 0) 909–916. [26] T. Darville, T.J. Hiltke, Pathogenesis of genital tract disease due to Chlamydia trachomatis, J. Infect. Dis. 201 (Supplement 2) (2010) S114–S125. [27] N. Reveneau, D.D. Crane, E. Fischer, H.D. Caldwell, Bactericidal activity of first-choice antibiotics against gamma interferon-induced persistent infection of human epithelial cells by chlamydia trachomatis, Antimicrob. Agents Chemother. 49 (5) (2005) 1787–1793, doi:10.1128/AAC.49.5.1787-1793.2005. [28] M. Singla, Role of tryptophan supplementation in the treatment of Chlamydia, Med. Hypotheses 68 (2) (2007) 278–280. [29] S.K. Schmidt, S. Siepmann, K. Kuhlmann, H.E. Meyer, S. Metzger, S. Pudelko, M. Leineweber, W. Däubener, Influence of tryptophan contained in 1-methyl-tryptophan on antimicrobial and immunoregulatory functions of indoleamine 2, 3-dioxygenase, PloS One 7 (9) (2012) e44797. [30] J.A. Ibana, R.J. Belland, A.H. Zea, D.J. Schust, T. Nagamatsu, Y.M. AbdelRahman, D.J. Tate, W.L. Beatty, A.A. Aiyar, A.J. Quayle, Inhibition of indoleamine 2,3-dioxygenase activity by levo-1-methyl tryptophan blocks gamma interferon-induced chlamydia trachomatis persistence in human epithelial cells, Infect. Immun. 79 (11) (2011) 4425–4437, doi:10.1128/IAI.05659-11. [31] V.D. Nikitushkin, G.R. Demina, A.S. Kaprelyants, Rpf proteins are the factors of reactivation of the dormant forms of actinobacteria, Biochemistry (Moscow) 81 (13) (2016) 1719–1734, doi:10.1134/S0 0 06297916130095. [32] F. Wolschendorf, A. Duverger, J. Jones, F.H. Wagner, J. Huff, W.H. Benjamin, M.S. Saag, M. Niederweis, O. Kutsch, Hit-and-Run Stimulation: a Novel Concept To Reactivate Latent HIV-1 Infection without Cytokine Gene Induction, Journal of Virology 84 (17) (2010) 8712–8720, doi:10.1128/JVI.00523-10. [33] F. Ye, J. Karn, Bacterial short chain fatty acids push all the buttons needed to reactivate latent viruses, Stem Cell Epigenetics 2 (1) (2015) e532. [34] C. Pedroza-Roldán, M.A. Flores-Valdez, Recent mouse models and vaccine candidates for preventing chronic/latent tuberculosis infection and its reactivation, Pathogens Dis. 75 (6) (2017), doi:10.1093/femspd/ftx079. [35] J.C. Burnett, K.-i. Lim, A. Calafi, J.J. Rossi, D.V. Schaffer, A.P. Arkin, Combinatorial latency reactivation for HIV-1 subtypes and variants, J. Virol. 84 (12) (2010) 5958–5974, doi:10.1128/JVI.00161-10. [36] L. Geeraert, G. Kraus, R.J. Pomerantz, Hide-and-seek: The challenge of viral persistence in HIV-1 infection, Annu. Rev. Med. 59 (1) (2008) 487–501, PMID: 17845138, doi:10.1146/annurev.med.59.062806.123001. [37] A. Bosque, K.A. Nilson, A.B. Macedo, A.M. Spivak, N.M. Archin, R.M. Van Wagoner, L.J. Martins, C.L. Novis, M.A. Szaniawski, C.M. Ireland, et al., Benzotriazoles reactivate latent HIV-1 through inactivation of stat5 sumoylation, Cell Rep. 18 (5) (2017) 1324–1334. [38] S. Chemicals, Indoximod (NLG-8189), 2013, (Available at: http://www.selleckchem.com/products/indoximod- nlg- 8189.html). Accessed October 12, 2017. [39] R.J. Conrado, J.D. Varner, M.P. DeLisa, Engineering the spatial organization of metabolic enzymes: mimicking nature’s synergy, Curr. Opin. Biotechnol. 19 (5) (2008) 492–499, Tissue, cell and pathway engineering, doi:10.1016/j.copbio.2008.07.006. [40] J. Becker, C. Wittmann, Bio-based production of chemicals, materials and fuels - corynebacterium glutamicum as versatile cell factory, Curr. Opin. Biotechnol. 23 (4) (2012) 631–640, Nanobiotechnology Systems biology, doi:10.1016/j.copbio.2011.11.012. [41] S.G. Cady, M. Sono, 1-methyl-dl-tryptophan, β -(3-benzofuranyl)-dl-alanine (the oxygen analog of tryptophan), and β -[3-benzo(b)thienyl]-dl-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase, Arch. Biochem. Biophys. 291 (2) (1991) 326–333, doi:10. 1016/0 0 03-9861(91)90142-6. [42] D.P. Wilson, Mathematical modelling of Chlamydia, ANZIAM 45 (2004) C201–C214. [43] P. van den Driessche, J. Watmough, Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission, Math. Biosci. 180 (2002) 29–48. 28 M.D. Akinlotan, D.G. Mallet and R.P. Araujo / Applied Mathematics and Computation 375 (2020) 124899 [44] S. Marino, I.B. Hogue, C.J. Ray, D.E. Kirschner, A methodology for performing global uncertainty and sensitivity analysis in systems biology, J. Theor. Biol. 254 (1) (2008) 178–196. [45] A. Hoare, D.G. Regan, D.P. Wilson, Sampling and sensitivity analyses tools (SaSAT) for computational modelling, Theor. Biol. Med. Modell. 5 (1) (2008) 4-4. [46] S.M. Blower, H. Dowlatabadi, Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example, Int. Stat. Rev. / Revue Internationale de Statistique 62 (2) (1994) 229–243. [47] L.S. Pontryagin, V.G. Boltyanskiy, R.V. Gamkrelidze, E.F. Mishchenko, The Mathematical Theory of Optimal Processes, Wiley, New Jersey, 1962. [48] S. Aniţa, V. Arnăutu, V. Capasso, An Introduction to Optimal Control Problems in Life Sciences and Economics: From Mathematical Models to Numerical Simulation with MATLAB®, Springer Science & Business Media, 2011. [49] S. Lenhart, J.T. Workman, Optimal control applied to biological models, CRC Press, London, UK, 2007. [50] K. Fister, S. Lenhart, J. McNally, Optimizing chemotherapy in an HIV model, Electron. J. Differ. Equ. 1998 (1998) 1–12. [51] J.M. Tchuenche, S.A. Khamis, F.B. Agusto, S.C. Mpeshe, Optimal control and sensitivity analysis of an influenza model with treatment and vaccination, Acta Biotheor. 59 (1) (2010) 1–28, doi:10.1007/s10441- 010- 9095- 8. [52] A.-M. Croicu, Short- and long-term optimal control of a mathematical model for HIV infection of cd4+ t cells, Bull. Math. Biol. 77 (11) (2015) 1–37. [53] O. Sharomi, T. Malik, Optimal control in epidemiology, Ann. Oper. Res. 251 (1–2) (2015) 1–17. [54] E. Bonyah, M. Khan, K. Okosun, J. G’omez-Aguilar, Modelling the effects of heavy alcohol consumption on the transmission dynamics of gonorrhea with optimal control, Math. Biosci. 309 (2019) 1–11, doi:10.1016/j.mbs.2018.12.015. [55] M. Khan, S.W. Shah, S. Ullah, J. G’omez-Aguilar, A dynamical model of asymptomatic carrier zika virus with optimal control strategies, Nonlinear Anal. Real World Appl. 50 (2019) 144–170, doi:10.1016/j.nonrwa.2019.04.006. [56] D. Kirschner, S. Lenhart, S. Serbin, Optimal control of the chemotherapy of HIV, J. Math. Biol. 35 (7) (1997) 775–792. [57] H.R. Joshi, Optimal control of an HIV immunology model, Optim. Control Appl. Methods 23 (4) (2002) 199–213, doi:10.1002/oca.710. [58] J. Karrakchou, M. Rachik, S. Gourari, Optimal control and infectiology: application to an HIV/AIDS model, Appl. Math. Comput. 177 (2) (2006) 807–818, doi:10.1016/j.amc.2005.11.092. [59] F. Agusto, Optimal chemoprophylaxis and treatment control strategies of a tuberculosis transmission model, World J. Modell. Simul. 5 (3) (2009) 163–173. [60] M. Martcheva, An Introduction to Mathematical Epidemiology, 61, Springer, 2015. [61] W. Fleming, R. Rishel, Deterministic and Stochastic Optimal Control, Springer New York, New York, NY, pp. 60–79. 10.1007/978-1-4612-6380-7_3 [62] E. Coddington, N. Levinson, Theory of Ordinary Differential Equations, Mc-Graw Hill Co., Inc., 1955. [63] P. Deuflhard, F. Bornemann, Scientific Computing with Ordinary Differential Equations, Texts in Applied Mathematics, 42, Springer, Berlin, 2002. [64] E. Coddington, An Introduction to Ordinary Differential Equations, Prentice-Hall Inc., 1961. [65] K. Krõlov, J. Frolova, O. Tudoran, J. Suhorutsenko, T. Lehto, H. Sibul, I. Mäger, M. Laanpere, I. Tulp, Ü. Langel, Sensitive and rapid detection of Chlamydia trachomatis by recombinase polymerase amplification directly from urine samples, J. Mol. Diagnos. 16 (1) (2014) 127–135. [66] A.P. Craig, R.G. Rank, A.K. Bowlin, H. Wand, D.P. Wilson, Target cell limitation constrains chlamydial load in persistent infections: results from mathematical modelling applied to mouse genital tract infection data, Pathogens Dis. 0 (2014) 1–8, doi:10.1111/2049-632X.12203. [67] H. Averette, Autoradiographic analysis of cell proliferation kinetics in human genital tissues, Obstet. Gynecol. (New York. 1953) (0029-7844) 31 (4) (1968) 580. [68] I.L. Cameron, {CHAPTER} 3 - cell proliferation and renewal in the mammalian body∗ , in: I.L. CAMERON, J.D. THRASHER (Eds.), Cellular and Molecular Renewal in the Mammalian Body, Academic Press, 1971, pp. 45–85. [69] M. Mcasey, L. Mou, W. Han, Convergence of the forward-backward sweep method in optimal control, Comput. Optim. Appl. 53 (1) (2012) 207–226, doi:10.1007/s10589- 011- 9454- 7. [70] B. Chachuat, Nonlinear and dynamic optimization: from theory to practice, Technical Report, Laboratoire d’Automatique, Ecolé Polytechnique Flédlérale de Lausanne, 2007. Available at: https://infoscience.epfl.ch/record/111939/files/Chachuat_07(IC32).pdf [71] MathWorks, fmincon, (Available at: https://au.mathworks.com/help/optim/ug/fmincon.html). Accessed January 20, 2017. [72] B. Chachuat, Optimal control, 2009, (Available at: http://la.epfl.ch/files/content/sites/la/files/shared/import/migration/IC_32/oc-3_solution.pdf). Accessed December 21, 2016. [73] W.L. Beatty, R.P. Morrison, G.I. Byrne, Reactivation of persistent Chlamydia trachomatis infection in cell culture., Infect. Immun. 63 (1) (1995) 199–205. [74] R.J. Belland, D.E. Nelson, D. Virok, D.D. Crane, D. Hogan, D. Sturdevant, W.L. Beatty, H.D. Caldwell, Transcriptome analysis of chlamydial growth during ifn-γ -mediated persistence and reactivation, Proc. Natl. Acad. Sci. 100 (26) (2003) 15971–15976, doi:10.1073/pnas.2535394100. [75] J.S. Hocking, L.A. Vodstrcil, W.M. Huston, P. Timms, M.Y. Chen, K. Worthington, R. McIver, S.N. Tabrizi, on behalf of the Australian Chlamydia Treatment Study (ACTS) investigators, A cohort study of Chlamydia trachomatistreatment failure in women: a study protocol, BMC Infect. Dis. 13 (1) (2013). 379–379 [76] CDC, Centers for Disease Control and Prevention: Sexually Transmitted Disease Surveillance 2013, Technical Report, CDC, 2014. Available at: http: //www.cdc.gov/std/stats13/surv2013-print.pdf. [Accessed January 22, 2014]. [77] W.E. Stamm, Azithromycin in the treatment of uncomplicated genital chlamydial infections, The American Journal of Medicine 91(3) (1991) 19S–22S. Suppl. 3A [78] A.L. Mellor, H. Lemos, L. Huang, Indoleamine 2,3-dioxygenase and tolerance: where are we now? Front. Immunol. 8 (2017) 1360, doi:10.3389/fimmu. 2017.01360. [79] O. Steingrimsson, J.H. Olafsson, H. Thorarinsson, R.W. Ryan, R.B. Johnson, R.C. Tilton, Azithromycin in the treatment of sexually transmitted disease, J. Antimicrob. Chemother. 25 (1990) 109–114. Suppl. A. [80] N. Larson, H. Ghandehari, Polymeric conjugates for drug delivery, Chem. Mater. 24 (5) (2012) 840–853, doi:10.1021/cm2031569. PMID: 22707853 [81] W.B. Liechty, D.R. Kryscio, B.V. Slaughter, N.A. Peppas, Polymers for drug delivery systems, Annu. Rev. Chem. Biomol. Eng. 1 (1) (2010) 149–173, PMID: 22432577, doi:10.1146/annurev- chembioeng- 073009- 100847. [82] M.J. Vicent, L. Dieudonné, R.J. Carbajo, A. Pineda-Lucena, Polymer conjugates as therapeutics: future trends, challenges and opportunities, Expert Opin. Drug Deliv. 5 (5) (2008) 593–614, PMID: 18491984, doi:10.1517/17425247.5.5.593. [83] L. Yeruva, S. Melnyk, N. Spencer, A. Bowlin, R.G. Rank, Differential susceptibilities to azithromycin treatment of chlamydial infection in the gastrointestinal tract and cervix, Antimicrob. Agents Chemother. 57 (12) (2013) 6290–6294, doi:10.1128/AAC.01405-13. [84] L. Yeruva, N. Spencer, A.K. Bowlin, Y. Wang, R.G. Rank, Chlamydial infection of the gastrointestinal tract: a reservoir for persistent infection, Pathogens Dis. 68 (3) (2013) 88–95. [85] R.G. Rank, L. Yeruva, Hidden in plain sight: chlamydial gastrointestinal infection and its relevance to persistence in human genital infection, Infect. Immun. 82 (4) (2014) 1362–1371. [86] J.K. Hale, Ordinary differential equations, Pure and Applied Mathematics XXI, Wiley-Interscience [John Wiley and Sons], New York (1969). [87] J. La Salle, The Stability of Dynamical Systems, Society for Industrial and Applied Mathematics, 1976, doi:10.1137/1.9781611970432.