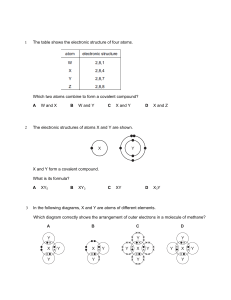
Resume Chemistry Learning In Secondary School “Alkana and Structure Atom” Dosen Pengampu: Ardi Widhia Sabekti, S.Pd., M.Pd DISUSUN OLEH: Reni Maharani 170384204040 PROGRAM STUDI PENDIDIKAN KIMIA FAKULTAS KEGURUAN DAN ILMU PENDIDIKAN UNIVERSITAS MARITIM RAJA ALI HAJI TANJUNGPINANG 2020 Resume of Fakhruddin Ar-razi a. Definition Atom Atom defined as particle The smallest from something Theory that no could be dividedfor again, will but from result invention next evidently atom still consist on particles that more small again that called particle subatomic. Particle The sub-atomic is: 1. Proton 2. Neutron 3. Electron. From the findings, it turns atom consists of particles Its simple, the so-called sub-atomic particles. Subatomic particles are: protons, electrons,and neutrons. Electrons are atomic particles were first discovered, the electrons found By Joseph John Thomsona British scientist.Proton constitute particle charged positive, position located in the nuclei. The existence of protons in the atomic nucleus was not alone. b. Struktur Of Atom With the discovery of the structure of atoms, then the difference between the atoms one by others can be explained. The difference is caused by differences in composition, ie the number of protons, electrons, and neutrons. In connection with the arrangement of atoms, we need to understand some terms, namely: 1. Atomic Number 2. Number Mass 3. Natation Atomic Strukture A question from fahrian bachri to fahruddin arrazi is how to calculated the number of electron and neutron of O2I as a participant only answered greetings and helped Razi in reading one ofe the slides. Resume of Mar’Atus Sholeha Alcene is a hydrocarbon coumponds that have a double bond (C=C) and are usually also called an unsaturated coumpound. As For the Structure of the alkenes is brounched structure alkenes, and unbranch alkene structure Characteristic of unsaturated hydrocarbon coumbonds: a. Alkene Contains a double bond between the carbon atoms b. Very reactives, and c. having Addiction reaction Liquid bromine test can distinguish saturated or unsaturated hydrocarbonds: Alkenes, don’t react with liquid bromine, alkene and alkyne react with liquid bromine is characteristic by the appearance. A question from Fahrian Bachri is what is the differences between alkane, alkene, and akune? Mar’Atus Sholeha answer : Alkane is a saturated hydrocarbon which means they are coumpounds with a single bond between, alkenses are unsaturated hydrocarbonds which means they are coumpounds with one or more double bonds between carbon atoms, while alkuna is also unsaturated hydrocarbonds, they have one or more threefold bonds between carbon atoms