Atomic structure
advertisement

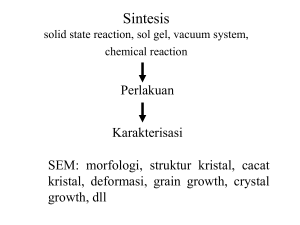
S.MORRIS 2006 PENDAHULUAN Definisi • Atom adalah partikel terkecil dari suatu elemen. • Setiap elemen memiliki struktur atom yang unik. • Menurut model atom Bohr, atom terdiri dari inti atom (nucleus) yang dikelilingi oleh elektron. • Nucleus terdiri dari proton (positif) dan neutron (netral). • Elektron yang mengelilingi inti atom berupa negatif. Nomor Atom • Semua elemen diurutkan dalam tabel periodik sesuai dengan nomor atomnya. Contoh : Hidrogen memiliki nomor atom 1 Helium memiliki nomor atom 2 HISTORY OF THE ATOM 460 BC Democritus develops the idea of atoms he pounded up materials in his pestle and mortar until he had reduced them to smaller and smaller particles which he called ATOMA (greek for indivisible) HISTORY OF THE ATOM 1808 John Dalton suggested that all matter was made up of tiny spheres that were able to bounce around with perfect elasticity and called them ATOMS HISTORY OF THE ATOM 1898 Joseph John Thompson found that atoms could sometimes eject a far smaller negative particle which he called an ELECTRON HISTORY OF THE ATOM 1904 Thompson develops the idea that an atom was made up of electrons scattered unevenly within an elastic sphere surrounded by a soup of positive charge to balance the electron's charge like plums surrounded by pudding. PLUM PUDDING MODEL HISTORY OF THE ATOM 1910 Ernest Rutherford oversaw Geiger and Marsden carrying out his famous experiment. they fired Helium nuclei at a piece of gold foil which was only a few atoms thick. they found that although most of them passed through. About 1 in 10,000 hit HISTORY OF THE ATOM helium nuclei gold foil helium nuclei They found that while most of the helium nuclei passed through the foil, a small number were deflected and, to their surprise, some helium nuclei bounced straight back. – Most of the positively charged “bullets” passed right through the gold atoms in the sheet of gold foil without changing course at all. – Some of the positively charged “bullets,” however, did bounce away from the gold sheet as if they had hit something solid. He knew that positive charges repel positive charges. HISTORY OF THE ATOM Rutherford’s new evidence allowed him to propose a more detailed model with a central nucleus. He suggested that the positive charge was all in a central nucleus. With this holding the electrons in place by electrical attraction However, this was not the end of the story. HISTORY OF THE ATOM 1913 Niels Bohr studied under Rutherford at the Victoria University in Manchester. Bohr refined Rutherford's idea by adding that the electrons were in orbits. Rather like planets orbiting the sun. With each orbit only able to contain a set number of electrons. Bohr’s Atom electrons in orbits nucleus HELIUM ATOM Shell proton + - N N + electron What do these particles consist of? - neutron ATOMIC STRUCTURE Particle Charge Mass proton + ve charge 1 neutron No charge 1 electron -ve charge nil ATOMIC STRUCTURE He 2 4 Atomic number the number of protons in an atom Atomic mass the number of protons and neutrons in an atom number of electrons = number of protons ATOMIC STRUCTURE Electrons are arranged in Energy Levels or Shells around the nucleus of an atom. • first shell a maximum of 2 electrons • second shell a maximum of 8 electrons • third shell a maximum of 8 electrons ATOMIC STRUCTURE There are two ways to represent the atomic structure of an element or compound; 1. 2. Electronic Configuration Dot & Cross Diagrams ELECTRONIC CONFIGURATION With electronic configuration elements are represented numerically by the number of electrons in their shells and number of shells. For example; Nitrogen 2 in 1st shell 5 in 2nd shell configuration = 2 , 5 2 + 5 = 7 N 7 14 ELECTRONIC CONFIGURATION Write the electronic configuration for the following elements; a) Ca 20 b) Na 40 2,8,8,2 d) Cl 17 35 2,8,7 11 23 c) 2,8,1 e) Si 14 28 2,8,4 O 8 16 2,6 f) B 5 11 2,3 DOT & CROSS DIAGRAMS With Dot & Cross diagrams elements and compounds are represented by Dots or Crosses to show electrons, and circles to show the shells. For example; X Nitrogen X X N XX X X N 7 14 DOT & CROSS DIAGRAMS Draw the Dot & Cross diagrams for the following elements; X 8 17 X a) O b) Cl 35 X 16 X X X X X X X X X Cl X X X X X O X X X X X X X X X Wave Model The Wave Model • Today’s atomic model is based on the principles of wave mechanics. • According to the theory of wave mechanics, electrons do not move about an atom in a definite path, like the planets around the sun. The Wave Model • In fact, it is impossible to determine the exact location of an electron. The probable location of an electron is based on how much energy the electron has. • According to the modern atomic model, at atom has a small positively charged nucleus surrounded by a large region in which there are enough electrons to make an atom neutral. Electron Cloud: • A space in which electrons are likely to be found. • Electrons whirl about the nucleus billions of times in one second • They are not moving around in random patterns. • Location of electrons depends upon how much energy the electron has. Electron Cloud: • Depending on their energy they are locked into a certain area in the cloud. • Electrons with the lowest energy are found in the energy level closest to the nucleus • Electrons with the highest energy are found in the outermost energy levels, farther from the nucleus. Indivisible Electron Greek X Dalton X Nucleus Thomson X Rutherford X X Bohr X X Wave X X Orbit Electron Cloud X X PARTIKEL BERMUATAN • Muatan negatif elektron yaitu 1.6 x 10-19 Coulomb • Massa elektron yaitu 9.11 x 10-31 kg • Muatan ion positif : suatu kelipatan dari muatan elektron dengan tanda muatan yang berlawanan. • Partikel yang diionisasikan tunggal, besar muatannya sama dengan muatan elektron. • Partikel yang diionisasikan rangkap, muatan ion menjadi dua kali muatan elektron. • Massa suatu atom sama dengan berat atom dikalikan dengan 1.66 x 10-27 kg. • Jari – jari elektron kira-kira 10-15 m , jari – jari atom 10-10 m SIFAT DASAR ELEKTRON • Elektron bergerak mengelilingi inti atom. • Elektron bermuatan negatif. • Elektron bergerak dengan arah yang sama dan menghasilkan arus listrik. • Elektron direpresentasikan dengan e-. • Elektron yang letaknya jauh dari nucleus memiliki energi yang lebih besar dibandingkan elektron yang berada dekat dengan nucleus. SUMMARY 1. The Atomic Number of an atom = number of protons in the nucleus. 2. The Atomic Mass of an atom = number of Protons + Neutrons in the nucleus. 3. The number of Protons = Number of Electrons. 4. Electrons orbit the nucleus in shells. 5. Each shell can only carry a set number of electrons.