Uploaded by
common.user151623
Hypothalamic Nutrient Sensing in Fish: Glucose, Fatty Acids, Amino Acids
advertisement

© 2024. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2024) 227, jeb247410. doi:10.1242/jeb.247410 REVIEW Hypothalamic integration of nutrient sensing in fish José L. Soengas*, Sara Comesana, ̃ Marta Conde-Sieira and Ayelé n M. Blanco The hypothalamus plays a crucial role in regulating feeding behavior in fish. In this Review, we aim to summarise current knowledge on specific mechanisms for sensing glucose, fatty acids and amino acids in fish, and to consider how this information is integrated in the hypothalamus to modulate feed intake. In fish, specific neuronal populations in the nucleus lateralis tuberalis (NLTv) of the hypothalamus are equipped with nutrient sensors and hormone receptors, allowing them to respond to changes in metabolite levels and hormonal signals. These neurons produce orexigenic (Npy and Agrp) and anorexigenic (Pomc and Cart) neuropeptides, which stimulate and suppress appetite, respectively. The modulation of feeding behavior involves adjusting the expression of these neuropeptides based on physiological conditions, ultimately influencing feeding through reciprocal inhibition of anorexigenic and orexigenic neurons and signalling to higher-order neurons. The activation of nutrient sensors in fish leads to an enhanced anorexigenic effect, with downregulation of agrp and npy, and upregulation of cart and pomc. Connections between hypothalamic neurons and other populations in various brain regions contribute to the intricate regulation of feeding behaviour in fish. Understanding how feed intake is regulated in fish through these processes is relevant to understanding fish evolution and is also important in the context of aquaculture. KEY WORDS: Feed intake, Fish, Hypothalamus, Nutrient sensing Introduction Much of our current understanding of the regulation of feeding behaviour is based on work in mammals, particularly lab models like mice and rats, with scarce information available in wild species. In mammals, food intake is regulated within the central nervous system (CNS) according to the levels of circulating nutrients, such as glucose, fatty acids and amino acids. These nutrients reflect the composition of the consumed food, and they act as a signal of the energy status of the organism. To detect changes in the levels of these circulating nutrients, a combination of enzymes, channels, carriers, transporters and other molecules in the brain act as sensors, enabling the detection and metabolism of nutrients. This information is integrated in the hypothalamus to elicit changes in food intake through changes in specific neuropeptides. Nutrients interact with circulating hormones, all acting on the hypothalamus (see Box 1). However, the focus of this Review is on the nutrient part of the regulation, rather than on hormonal effects. In fish, research carried out in recent years has provided evidence for the existence of comparable mechanisms to those known in Centro de Investigació n Marina, ̃ Laboratorio de Fisioloxı́a Animal, Departamento de Bioloxı́a Funcional e Ciencias da Saú de, Facultade de Bioloxı́a, Universidade de Vigo, 36310 Vigo, Spain. *Author for correspondence ( [email protected]) J.L.S., 0000-0002-6847-3993; S.C., 0000-0002-3020-8377; M.C., 0000-00029763-6202; A.M.B., 0000-0002-7683-4154 mammals. In this Review, we aim to describe these processes in fish, focusing not only on specific mechanisms for sensing glucose, fatty acids and amino acids, but also discussing their impact on metabolism and the regulation of feed intake. It is our hope that this synthesis will allow us to identify key knowledge gaps that can be addressed by future research. It is important to mention that fishes form a heterogeneous group, and that, so far, knowledge on the mechanisms underlying their nutrient sensing has been obtained from a relatively small number of fish species. Therefore, it is not currently possible to suggest clear trends within teleost fish regarding these mechanisms. Glucosensing Mechanisms In fish, there are multiple hypothalamic mechanisms for detecting glucose levels, and most of them, as characterized in mammals, are dependent on the entry of glucose into the cells and its subsequent metabolism. The best-known glucosensing mechanism is based on glucokinase (GCK) activity and GLUT2 (SLC2a2)-mediated transport (Fig. 1; Marty et al., 2007; De Backer et al., 2016). Once glucose is transported into a neuron through the low-affinity GLUT2 transporter, GCK facilitates its phosphorylation to glucose6-phosphate. This initiates glycolysis, increasing intracellular ATP levels and leading to the closure of ATP-sensitive potassium (K+ ATP) channels. This closure induces membrane depolarization, allowing calcium influx through L-type voltage-dependent calcium channels, and ultimately enhancing neuronal activity. In fish, researchers have used a variety of techniques to alter glucose levels experienced by rainbow trout (Oncorhynchus mykiss) hypothalamic cells, both in vitro and in vivo (Polakof and Soengas, 2008; Polakof et al., 2007a, 2007b, 2008a,b,c; Conde-Sieira et al., 2010a,b, 2011, 2012; Aguilar et al., 2011; Otero-Rodiño et al., 2015a). All such interventions lead to modifications in different components involved in the Gck–Glut2-dependent glucosensing mechanism within the hypothalamus. The expression of the components of this system are increased when circulating levels of glucose rise and decreased when blood glucose levels fall. In addition to rainbow trout, there is evidence for central glucosensing capacity in other fish species, such as medaka (Oryzias latipes, Hasebe et al., 2016) or grass carp (Ctenopharyngodon idella, Chen et al., 2022). As noted above, there are multiple glucosensing mechanisms, and not all rely on Gck–Glut2. In mammals, an alternative mechanism relies on the transport of glucose into the neuron by the sodium-coupled glucose cotransporter 1 (SGLT-1), dependent on the entry of Na+ (Fig. 1; O’Malley et al., 2006). Because glucose is electrically neutral, the entry of Na+ through SGLT-1 is sufficient to induce depolarization and increase neuronal activity. This response can occur either through direct changes of the membrane potential or indirectly through coupling with a G-protein (DíezSampedro et al., 2003). In addition to stimulating neuronal activity, elevated glucose levels lead to increased levels of Sglt1 mRNA in various mammalian tissues, meaning that this transporter effectively serves as a glucosensor (O’Malley et al., 2006; González et al., 2009). 1 Journal of Experimental Biology ABSTRACT Journal of Experimental Biology (2024) 227, jeb247410. doi:10.1242/jeb.247410 List of abbreviations Acc Acly ACS ALA Ampk BCAA Bcat Bckdh Bckdk Bsx Cart Cck CNS Cpt-1 Creb ER FA FA-CoA Fas Fat/cd36 Fatp4 Fbpase Foxo1 Gck Gcn2 Gdh Ghrl Glp-1 Gls Glut2 Gpase Gpr Gsase ICV IP IP3 K+ ATP Lat1 LCFA Ldh Lpl Lxr Mcd MCFA mTOR NAT NLTd NLTl NLTv NPO Npy Pkc Plc Pomc Pparα Pyy ROS S6 SCFA Sesn2 Sglt-1 Snat2 Srebp1c T1r1–Tlr3 Ucp2 Vdcc Vm acetyl-CoA carboxylase ATP citrate lyase acyl-CoA synthase α-linolenate AMP-activated protein kinase branched-chain amino acid. branched-chain aminotransferase branched-chain alpha-keto acid dehydrogenase complex branched-chain ketoacid dehydrogenase kinase brain homeobox transcription factor cocaine- and amphetamine-regulated transcript cholecystokinin central nervous system carnitine palmitoyltransferase 1 cAMP response-element binding protein endoplasmic reticulum fatty acid fatty acid-coenzyme A fatty acid synthase fatty acid transporter CD36 fatty acid transporter 4 fructose 1,6-bisphosphatase forkhead box O1 glucokinase general control nonderepressible 2 glutamate dehydrogenase ghrelin glucagon-like peptide 1 glutamine synthase facilitated glucose carrier type 2 glycogen phosphorylase G-protein-coupled receptor glycogen synthase intracerebroventricular intraperitoneal inositol triphosphatase inward rectifier ATP-dependent K+ channel L-type amino acid transporter 1 long-chain fatty acid lactate dehydrogenase lipoprotein lipase liver X receptor malonyl CoA dehydrogenase medium-chain fatty acid mechanistic target of rapamycin anterior tuber nucleus lateral tuber nucleus pars dorsalis lateral tuber nucleus pars lateralis lateral tuber nucleus pars ventralis preoptic nuclei neuropeptide Y protein kinase C phospholipase C pro-opiomelanocortin peroxisome proliferator-activated receptor type α peptide tyrosine-tyrosine reactive oxygen species ribosomal protein S6 short-chain fatty acid sestrin 2 sodium/glucose linked transporter 1 sodium-dependent neutral amino acid transporter 2 sterol regulatory element-binding protein type 1c taste receptor type 1 member 1–3 uncoupling protein 2 L-type voltage-dependent calcium channel membrane potential Glossary Anorexigenic A substance that reduces appetite, resulting in reduced food consumption. Blood–brain barrier A semipermeable border of endothelial cells that regulates the transfer of solutes and chemicals between the circulatory system and the central nervous system. Brockmann body An endocrine organ in some teleost fish composed of a collection of pancreatic tissues. Hypothalamus–pituitary–inter-renal axis A set of direct influences and feedback interactions among three components present in teleost fish: the hypothalamus (a part of the brain located below the thalamus), the pituitary gland (a pea-shaped structure located below the hypothalamus) and the inter-renal tissue (in the middle of the head kidney). These organs and their interactions constitute the HPI axis. Metabotropic receptor A type of membrane receptor that initiates several metabolic steps to modulate cell activity. Orexigenic A substance that increases appetite and stimulates feeding. Reactive oxygen species Highly reactive chemicals that can be produced from oxygen, water or hydrogen peroxide. β-oxidation A catabolic process by which fatty acids are broken down to generate acetyl-CoA. In fish, Sglt-1 is present in various tissues, including the hypothalamus (rainbow trout: Sugiura et al., 2003; Soengas and Polakof, 2013; Conde-Sieira et al., 2013; Craig et al., 2013; gilthead sea bream, Sparus aurata: Sala-Rabanal et al., 2004), although its involvement in glucosensing is not clear. Another GCK-independent glucosensing mechanism involves the stimulation of the sweet taste receptor, which is a heterodimer of type 1 taste receptor subunits (T1Rs) formed by T1R2, T1R3 and αgustducin. Binding of glucose to this receptor induces the activation of an intracellular signalling cascade leading to membrane depolarization (Fig. 1; Ren et al., 2009; Herrera Moro Chao et al., 2016). T1R2/3 is a metabotropic (see Glossary) G-protein-coupled (GPR) receptor that can modulate neuronal activity in the presence of glucose in the brain (Welcome et al., 2015) in a manner similar to that of T1R2/3 located in taste buds (Kinnamon, 2012). In rainbow trout hypothalamus, the mRNA abundance of α-gustducin, t1r2 and t1r3 decreases under conditions of hyperglycemia, suggesting that the sweet taste receptor may play a role in glucosensing in this brain area (Otero-Rodiño et al., 2015a). Furthermore, levels of mRNA of t1r2 in the brain of rainbow trout increase in fish nutritionally programmed to cope with elevated levels of dietary carbohydrate (Balasubramanian et al., 2016). An alternative glucosensor system based on the nuclear liver X receptor (LXR) has been described in mammals. Here, the activation of LXR increases in response to increased glucose, resulting in a decrease in gluconeogenesis (Anthonisen et al., 2010) and changes in the mRNA abundance of certain transcription factors (Higuchi et al., 2012; Kim and Ahn, 2004; Festuccia et al., 2014). Glucosensing in mammals can also be mediated by enhanced mitochondrial production of reactive oxygen species (ROS; see Glossary) by electron leakage, which can occur through the mitochondrial uncoupling protein UCP2a (Blouet and Schwartz, 2010; Blanco de Morentin et al., 2011), such that an increase in 2 Journal of Experimental Biology REVIEW Box 1. Feeding control by hypothalamic neuropeptides The mammalian hypothalamus integrates signals originating from both the brain and peripheral organs, resulting in alterations in food intake. Two distinct neuronal populations within the arcuate nucleus are primarily responsible for signal integration: orexigenic (appetitestimulating) neurons, producing neuropeptide Y (NPY) and agoutirelated peptide (AgRP), and anorexigenic (appetite-suppressing) neurons, producing pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART). These neuronal populations are equipped with nutrient sensors and receptors for hormones regulating appetite. This enables them to respond to fluctuations in the levels of circulating metabolites – including fatty acids, glucose and amino acids – as well as hormonal signals. The subsequent response involves modulating the expression levels of Npy, Agrp, Pomc and Cart based on specific physiological conditions (Schwartz et al., 2000; Levin et al., 2004; Mobbs et al., 2005; Mountjoy et al., 2007; Fioramonti et al., 2007; Sohn, 2015). Ultimately, feeding behaviour is influenced by the reciprocal inhibition of orexigenic and anorexigenic neurons, and their signalling to higher-order neurons in diverse hypothalamic and extrahypothalamic regions (Blouet and Schwartz, 2010; Morton et al., 2014). In fish, Agrp/Npy and Pomc/Cart neurons are present in the nucleus lateralis tuberalis (NLTv; Soengas et al., 2018). The mRNA levels of these neuropeptides in fish are responsive to the feeding status (Bodas et al., 2023), indicating their pivotal role in the regulation of feed intake (Volkoff, 2016; Delgado et al., 2017). In fish, the activation of nutrient sensors results in an enhanced anorexigenic potential, achieved through the downregulation of agrp and npy expression and the upregulation of cart and pomc expression (Conde-Sieira and Soengas, 2017; Delgado et al., 2017; Soengas et al., 2018). The finding that pomc-knockout (KO) zebrafish (Danio rerio) display increased feed intake compared with the wild type also supports the involvement of Pomc as an anorexigenic neuropeptide in fish (Yang et al., 2023). Additionally, like the situation in mammals, Npy/Agrp and Pomc/Cart neurons establish connections with other neuronal populations in both hypothalamic and extra-hypothalamic areas in fish (Bodas et al., 2023), although the precise understanding of their neuropeptide production remains elusive (Soengas et al., 2018). UCP2a activity indicates a lower level of glucose (Kong et al., 2010; Beall et al., 2012; Thorens, 2012). In fish, there is some evidence that both the Lxr- and mitochondria-dependent mechanisms of glucosensing are functional in rainbow trout hypothalamus (OteroRodiño et al., 2015a, 2016). In recent years, experimental evidence from mammals has pointed to an important role for astrocytes and tanycytes in the glucosensing function of the hypothalamus (Pellerin and Magistretti, 1994; López-Gambero et al., 2019). In fish, there is little information on this mechanism, but lactate treatment can modulate central glucose metabolism in rainbow trout, which would suggest the possible presence of an astrocyte–neuron lactate shuttle that would facilitate glucosensing (Polakof and Soengas, 2008; Otero-Rodiño et al., 2015b). In summary, in fish, there is sufficient information available on the Glut2–Gck mechanism of glucosensing to suggest that it plays a comparable role in fish to that known in mammals. However, more fish species need to be assessed to in order to determine the relative importance of this mechanism in species with different dietary habits (e.g. carnivore, omnivore or herbivore) or habitats. In contrast, in fish, the information regarding alternative mechanisms of glucosensing is very scarce, demanding further research. Impact of glucosensing on feeding and metabolism Changes in glucose levels induce changes in feed intake and in appetite-related neuropeptides in fish (Polakof et al., 2011; Soengas, 2014). In general, the activation of glucosensing systems induced by Journal of Experimental Biology (2024) 227, jeb247410. doi:10.1242/jeb.247410 the presence of high levels of carbohydrate in the diet or by increasing levels of glucose in vivo or in vitro results in decreased feed intake, in parallel with an increased abundance of mRNA encoding the anorexigenic (see Glossary) neuropeptides Pomc and Cart, and decreased levels of mRNA encoding the orexigenic (see Glossary) neuropeptides Npy and Agrp (Table 1). Moreover, in regions of the brain where neuropeptides are generated, studies utilizing histochemistry reveal the existence of proteins associated with glucose sensing, as demonstratred for Gck (Polakof et al., 2009), as well as Sglt-1, Lxr or T1r3 (Otero-Rodiño et al., 2019a,b). These findings imply a functional connection between glucosensors and neuropeptides in these brain areas. As well as having a role in regulating appetite, glucosensing mechanisms are also involved in regulating other aspects of energy homeostasis: for example, glucosensing influences hormone secretion and energy expenditure, which modulate the activity of peripheral organs such as the liver and pancreas (Timper and Brüning, 2017; López-Gambero et al., 2019). In fish, intracerebroventricular (ICV) glucose administration affects hepatic metabolism (Polakof and Soengas, 2008). Therefore, the presence of glucose in the fish brain is a signal of energy abundance, which induces a reduction in the activity of the hepatic pathways involved in glucose production and release. In mammals, hypothalamic detection of high glucose levels induces pancreatic counter-regulatory responses to restore normal blood glucose levels. These responses are mainly mediated by parasympathetic and sympathetic efferent nerves that innervate pancreatic α- and β-cells, inducing the release of the hormones insulin and glucagon (Blouet and Schwartz, 2010; Ogunnowo-Bada et al., 2014; Roh et al., 2016). In rainbow trout, changes in glucose concentration in the brain result in increased Gck activity and expression in Brockmann bodies (see Glossary), which are homologous to the mammalian endocrine pancreas (Polakof and Soengas, 2008). Accordingly, in fasted fish, plasma insulin levels decrease and plasma glucagon levels increase (Navarro and Gutiérrez, 1995), whereas the expression of insulin is higher in zebrafish (Danio rerio) exposed to elevated glucose levels (Jurczyk et al., 2011). Although there is a reasonable amount of information available regarding the impact of hypothalamic glucosensing on feed intake in fish, the impact of central glucosensing on peripheral metabolism is basically unknown and requires further research, especially considering the impact of metabolic changes on fish growth. Fatty acid sensing Mechanisms The most-accepted mechanism through which fatty acids (particularly long-chain fatty acids; LCFAs) are sensed in mammalian hypothalamic cells is metabolic in nature. Increased plasma levels of LCFAs lead to an increase in the levels of malonyl-CoA, which in turn inhibits carnitine palmitoyltransferase 1 (CPT-1; also known as carnitine acyltransferase 1; Fig. 2). This enzyme is in the outer mitochondrial membrane, and it is responsible for catalyzing the transport of LCFAs into mitochondria. Inhibition of CPT-1 prevents the mitochondria from importing fatty acid-CoA for β-oxidation (see Glossary; López et al., 2005, 2007; Gao et al., 2013). This mechanism is also likely to be functional in fish. For example, hypothalamic cells of several fish species show increased levels of malonyl-CoA and/or decreased Cpt-1 in response to the LCFA oleate (C18:1 n-9), both in vitro and in vivo [rainbow trout: Librán-Pérez et al., 2012, 2013b, 2014a; Velasco et al., 2017b; Senegalese sole (Solea senegalensis): Conde-Sieira et al., 2015; Chinese perch (Siniperca chuatsi): Luo et al., 2020]. 3 Journal of Experimental Biology REVIEW REVIEW Journal of Experimental Biology (2024) 227, jeb247410. doi:10.1242/jeb.247410 1 Glucose Glucose Glucose 1 T1r2 Glucose Sgl Glut2 t-1 Gck Gsase Glycogen Glucose-6P 3 T1r3 Gpase Glucose Na+ 2 Vm Fructose-6P FBPase − Plc/IP3 pathway Glycolysis 4 Lxr + PPAR SREBP1c Ldh Pyruvate Lactate ER Nucleus Ca2+ Ca2+ Ca2+ Ucp2 1 ADP ATP K+ATP channel 5 Pyruvate ROS Acetyl-CoA NADH, FADH2 Respiratory chain Ca2+ Krebs cycle Vdcc ATP K+ Mitochondria Ca2+ Vm Unlike the situation in mammals, where fatty acid-sensing mechanisms only respond to some LCFAs, the fish hypothalamus seems to detect monounsaturated fatty acids, medium-chain fatty acids (MCFAs) and polyunsaturated fatty acids (PUFAs). For example, increased malonyl-CoA and/or decreased Cpt-1 levels in the fish hypothalamus occur not only in response to oleate, but also to the MCFA octanoate in rainbow trout (Librán-Pérez et al., 2012, 2013b, 2014a) and the PUFA α-linolenate (ALA) in Senegalese sole (CondeSieira et al., 2015). The ability of fish (especially marine species) to respond to changes in PUFA levels might relate to the high amount of n-3 PUFAs in fish diets (Sargent et al., 2002) and consequently in their tissues (Mourente and Tocher, 1992; Tocher, 2003). Alternative mechanisms of fatty acid sensing are also present in the fish hypothalamus. One such mechanism involves the fatty acid translocase (also known as cluster of differentiation 36; Fat/cd36; Fig. 2). This protein increases its capacity to bind to fatty acids in response to increased fatty acid levels, resulting in the modulation of mRNA abundance of several transcription factors, such as srebp1c and ppara (Le Foll et al., 2009). In fish, administration of oleate (which is not sensed by mammals) and/or octanoate upregulates the expression of hypothalamic mRNAs encoding Cd36, Srebp1c and Pparα in rainbow trout (Librán-Pérez et al., 2012, 2014a) and Chinese perch (Luo et al., 2020). A similar response also occurs upon feeding rainbow trout with a lipid-enriched diet (Librán-Pérez et al., 2015a). Similarly, an oleate-induced increase in abundance of cd36 mRNA and an oleate- and ALA-induced increase in ppara mRNA occur in the head of Senegalese sole post-larvae when the same nutrients are administered orally (Velasco et al., 2017a). Expression of cd36 is also upregulated in the brain of grass carp fed a high-fat diet (Tian et al., 2017) and in the hypothalamus of Chinese perch after ICV treatment with oleate, linolenate or αlinolelante (Luo et al., 2020). Intraperitoneal administration of oleate and ALA downregulate ppara and srebp1c in the Senegalese sole hypothalamus (Conde-Sieira et al., 2015). Finally, Fat/cd36 gene knockout suppresses the spontaneous linoleate preference in zebrafish (Liu et al., 2017). Another response of mammalian hypothalamic neurons to increased levels of LCFAs is the inhibition of K+ ATP channels (Fig. 2). This inhibition can be caused either by the activation of specific isoforms of protein kinase C (PKC) (Benoit et al., 2009) or by 4 Journal of Experimental Biology Fig. 1. Schematic summary of processes involved in glucosensing in the fish hypothalamus. A rise in circulating glucose levels is sensed through different mechanisms. (1) Increased transport of glucose by Glut2 leads to phosphorylation by Gck, initiating glycolysis, increasing intracellular ATP levels and leading to the closure of K+ ATP channels, which induces membrane depolarization, allowing calcium influx via Vdcc. (2) Transport of glucose into the neuron via Sglt-1 is dependent on the simultaneous entry of Na+, inducing depolarization. (3) Binding of glucose to T1r2–T1r3 induces activation of intracellular signalling, leading to membrane depolarization. (4) Activation of Lxr in response to increased glucose levels decreases gluconeogenesis and results in changes in transcription factors. (5) Increased levels of glucose enhance mitochondrial production of ROS by stimulating electron leakage. For definition of all symbols, see list of abbreviations. REVIEW Journal of Experimental Biology (2024) 227, jeb247410. doi:10.1242/jeb.247410 Table 1. Changes in feed intake and mRNA abundance of hypothalamic neuropeptides in different fish species exposed to different diets or experimental treatment with nutrients in vivo or in vitro Species Glucose/carbohydrates Increased glucose Catfish (Clarias gariepinus) Rainbow trout (Oncorhynchus mykiss) HC diet LC diet Fatty acids/fats Increased octanoate, linolenate or ALA Increased oleate, octanoate or ALA Increased fatty acids HF diet Decreased fatty acids Amino acids/proteins Increased leucine High leucine diet Increased valine Increased methionine or lysine HP diet Atlantic salmon (Salmo salar) European eel (Anguilla anguilla) Goldfish (Carassius auratus) Gilthead sea bream (Sparus auratus) Grass carp (Ctenopharyngodon idella) Nile tilapia (Oreochromis niloticus) Japanese flounder (Paralichthys olivaceus) Japanese seabass (Lateolabrax maculatus) Rainbow trout Neuropeptide mRNA ↑ cart ↓ npy ≈ agrp ↑ cart, pomc ≈ npy, cart ↓npy, agrp Rainbow trout Senegalese sole (Solea senegalensis) Atlantic salmon Chinook salmon (Oncorhynchus tshawytscha) Polka-dot grouper (Cromileptes altivelis) Rainbow trout Senegalese sole Rainbow trout Catfish hybrid (Pelteobagrus vachelli×Leiocassis longirostris) Chinese perch Rainbow trout Tiger puffer (Takifugu rubripes) Atlantic salmon Golden pompano (Trachinotus ovatus) Grouper (Epinephelus coioides) Chinese perch Rainbow trout Chinese perch ↓ ↓ ↓ Figueiredo-Silva et al., 2013 Liu et al., 2019** ↓ agrp Han et al., 2022 ↓ npy ↓ ↓ ↑ ↓ npy, agrp ↑ cart, pomc ↓ npy, agrp ↑ cart, pomc ↓ npy, agrp ↑ cart, pomc ↓ npy, cart, pomc ↓ npy, agrp ↑ cart, pomc ↑ npy, agrp ↓ cart, pomc ≈ npy, agrp, cart, pomc ↑ npy Rainbow trout Reference Subhedar et al., 2011 Ruibal et al., 2002; Polakof et al., 2007a, 2008a; Conde-Sieira et al., 2010a,b, 2012; Aguilar et al., 2011; Figuereido-Silva et al., 2012a,b; Otero-Rodiño et al., 2015a, 2016 Krogdahl et al., 2004 Suárez et al., 2002 Narnaware and Peter, 2002 Babaei et al., 2017; Basto-Silva et al., 2021 Chen et al., 2022 ↓ ↓ ↓ ↓ Siberian sturgeon (Acipenser baerii) Rainbow trout Chinese perch (Siniperca chuatsi) Feed intake Kaushik et al., 1989; Suárez et al., 2002; Polakof et al., 2008b,c; Figuereido-Silva et al., 2012a,b Gong et al., 2014 Sánchez-Muros et al., 1998; Capilla et al., 2003; Polakof et al., 2008b,c Luo et al., 2020; Feng et al., 2022 Librán-Pérez et al., 2012, 2014a; Velasco et al., 2016a,b, 2017a Conde-Sieira et al., 2015 ↓ ↓ Hevrøy et al., 2012 Silverstein et al., 1999 ↓ Williams et al., 2006 ↓ Peragón et al., 2000; Rasmussen et al., 2000; Gélineau et al., 2001; Forsman and Ruohonen, 2009; Saravanan et al., 2013; Librán-Pérez et al., 2015a Bonacic et al., 2016 Librán-Pérez et al., 2014b ↓ ↓ Zhao et al., 2020 ↓ ↓ Chen et al., 2021 Comesaña et al., 2018a,b ↓ ↓ ↓ Wei et al., 2022 Comesaña et al., 2021a; Lai et al., 2023 Tan et al., 2016 ↓ ↑ ↑ ↑ Niu et al., 2021 Chen et al., 2021 Comesaña et al., 2018a,b Zou et al., 2022 ↓ Skiba-Cassy et al., 2013; Basto-Silva et al., 2022 agrp, agouti-related peptide; cart, cocaine and amphetamine-related transcript; FI, feed intake; HC, high carbohydrate; HF, high fat; HP, high protein; LC, low carbohydrate; LF, low fat; npy, neuropeptide Y; pomc, pro-opio melanocortin; ↑, increase; ↓, decrease; ≈, no change. the enhanced production of ROS, owing to electron leakage from mitochondria (Schönfeld and Wojtczak, 2008). In the fish hypothalamus, changes in the mRNA abundance of components of the K+ ATP channel and/or mitochondrial uncoupling (through UCP2a) occur in response to various fatty acids in rainbow trout (Librán-Pérez et al., 2012, 2013b, 2014a; Velasco et al., 2016a,b, 2017b) or Senegalese sole (Conde-Sieira et al., 2015; Velasco et al., 2017c), thus supporting the role of this channel. Lipoprotein lipase (LPL) has also been identified as a lipid sensor in the mammalian hypothalamus. The activity of LPL increases in 5 Journal of Experimental Biology Treatment REVIEW Journal of Experimental Biology (2024) 227, jeb247410. doi:10.1242/jeb.247410 FAs Ffar1 Gpr84 FAs FAs 2 Fat/ cd36 Acetyl-CoA Fatp4 Acc-P Mcd Malonyl-CoA 5 Gpr119 6 ? 1 Fas FA Plc/IP3 pathway FA Lpl Acs FA-CoA (primarily LCFA-CoA) 3 Gene expression orexigenic/anorexigenic neuropeptides 1 Pkc Cp FA-CoA Ucp2 ADP ATP K+ATP channel ER Ca2+ t-1 Nucleus E-oxidation Acetyl-CoA (if excessive) ROS Respiratory chain 4 NADH, FADH2 Krebs cycle Ca2+ Ca2+ Ca2+ ATP K+ Mitochondria Vdcc Ca2+ Vm Fig. 2. Schematic summary of processes involved in fatty acid sensing in the fish hypothalamus. A rise in circulating levels of LCFA, MCFA or PUFA is sensed through different mechanisms. (1) Increased levels of malonyl-CoA, inhibiting Cpt-1. (2) Increased capacity of Fat/cd36 to bind to fatty acids, resulting in the modulation of Srebp1c and Pparα. (3) Inhibition of K+ ATP channels by the activation of specific isoforms of Pkc. (4) Inhibition of K+ ATP channels by enhanced production of ROS owing to electron leakage by mitochondria. (5) Increased Lpl activity. (6) Activation of Ffar1, Gpr84 and Gpr119, triggering the activation of Plc/IP3 signalling and ultimately leading to an increase in intracellular Ca2+ levels. For definition of all symbols, see list of abbreviations. knowledge on Gpr-mediated fatty acid sensing in the fish brain is still very scarce and more research on this topic is required. Impact of fatty acid sensing on feeding and metabolism Lipids have an important impact on feed-intake regulation in fish: fish fed lipid-enriched diets display different feed-intake levels (usually decreased) compared with those fed a normal diet. Moreover, enhanced lipid storage is also usually associated with reduced feed intake (Shearer et al., 1997; Silverstein et al., 1999; Johansen et al., 2002, 2003). Among the lipid pool, fatty acids are the most important lipids in terms of energy use; thus, it is not surprising that the differences in feeding observed with lipid-enriched diets are predominantly caused by fatty acids (Table 1). For example, in rainbow trout, the lowest feed intake is seen in fish fed with lipidenriched diets that result in higher plasma levels of fatty acids (Luo et al., 2014) and PUFAs (Roy et al., 2020). Moreover, a clear increase in feed intake is observed in rainbow trout displaying a pharmacologically mediated fall in circulating fatty acid levels (Librán-Pérez et al., 2014b). In addition, intraperitoneal (LibránPérez et al., 2012) or ICV (Librán-Pérez et al., 2014a; Velasco et al., 2016a,b) administration of oleate or octanoate results in a significant 6 Journal of Experimental Biology response to a rise in LCFA levels (Picard et al., 2014). It is not clear whether this lipid sensor is active in fish: in rainbow trout hypothalamus, the abundance of lpl mRNA decreases in response to ICV administration of oleate (Velasco et al., 2016a,b) or in vitro incubation with this fatty acid (Velasco et al., 2017b). Finally, it is well established that hypothalamic neurons in mammals express GPRs – particularly GPR40 and GPR120 [also named free fatty acid receptor 1 (FFAR1) and 4 (FFAR4), respectively] – that can detect and respond to LCFAs (Dragano et al., 2017). Activation of these receptors is enhanced as LCFA levels increase, triggering the activation of the phospholipase C (PLC)/inositol 1,4,5-triphosphate (IP3) intracellular signalling pathway, and ultimately leading to an increase in intracellular Ca2+ levels (Usui et al., 2019). The presence of GPR84, which binds to MCFAs, has also been reported in the mammalian brain (Ichimura et al., 2009). In contrast to mammals, Ffar4 is absent in fish; consequently, Ffar1 is the only putative receptor for LCFAs in fish (Fig. 2). Recent studies have provided evidence for the presence of fatty acid-sensing mechanisms involving Ffar1 in rainbow trout hypothalamus (Velasco et al., 2020), as well as for mechanisms involving Gpr84 and Gpr119 (Fig. 2; Velasco et al., 2021). However, decrease in feed intake in rainbow trout, with the effect being greater for octanoate. A decrease in abundance of npy/agrp mRNA and/or an increase in pomc/cart mRNA in response to feeding a lipid-enriched diet is observed in the hypothalamus of several fish species (Table 1). A similar increase also occurs in response to increased levels of specific fatty acids, such as oleate, octanoate or ALA (Table 1). The octanoate-mediated modulation of neuropeptide expression appears to be exclusive to fish; this fatty acid does not induce any change in mRNA abundance of neuropeptides in mammals (Hu et al., 2011). Apart from its effects on feeding, the hypothalamic detection of fatty acids impacts several peripheral metabolic processes involved in energy homeostasis, such as hepatic glucose production and lipogenesis (Obici et al., 2002; Morgan et al., 2004; Migrenne et al., 2011). In fish, several studies have demonstrated that ICV treatment with fatty acids also alters parameters related to glucose and lipid metabolism in peripheral tissues (including the liver and the Brockman bodies), as in mammals (Migrenne et al., 2011; CondeSieira and Soengas, 2017). Thus, ICV-administered oleate and octanoate lead to increased levels of glucose and glycogen and decreased levels of fatty acid and total lipid, as well as decreased activities of Gck, fructose 1,6-bisphosphatase (Fbpase), fatty acid synthase (Fas) and Cpt-1 in the liver of rainbow trout. These changes counter-regulate the elevated fatty acid levels as detected in the brain (Librán-Pérez et al., 2015c). The functional connection between central fatty acid sensing and production/release of fuels from the liver is likely to be mediated through the vagus and splanchnic nerves, which innervate the liver and the gastrointestinal tract (Burnstock, 1959; Seth and Axelsson, 2010). At least in rainbow trout, the hypothalamus–inter-renal–pituitary axis (see Glossary) has also been implicated in the counter-regulatory response of the liver to a fall in circulating fatty acid levels (Librán-Pérez et al., 2015c), in a manner comparable to that described in mammals (Oh et al., 2012, 2014). Regarding the Brockman bodies, ICV administration of oleate and octanoate in rainbow trout results in the modulation of parameters related to lipid metabolism in this tissue (Librán-Pérez et al., 2015b). In summary, lipid-enriched diets typically result in decreased feed intake in fish, likely owing to increased levels of fatty acids in the blood. Changes in feed intake are associated with changes in hypothalamic npy/agrp and pomc/cart expression, particularly in response to lipid-enriched diets or fatty acid administration. Moreover, hypothalamic detection of fatty acids influences peripheral metabolic processes, including glucose and lipid metabolism. However, further research on this subject is required to provide mechanistic detail in a wider range of fish species. Amino acid sensing Mechanisms In mammals, branched-chain amino acids (BCAAs), namely leucine, isoleucine and valine, emerge as particularly relevant in terms of nutrient sensing. This significance stems from the fact that postprandial plasma levels of most amino acids, except for BCAAs, remain stable (Heeley and Blouet, 2016). Notably, among the three BCAAs, only leucine appears to be detected in the hypothalamus (Morrison and Laeger, 2015; Heeley and Blouet, 2016). Furthermore, mammalian hypothalamic neurons involved in the regulation of food intake respond specifically to changes in leucine levels (Heeley et al., 2018). In teleost fish, the available data suggest that amino acid sensing is like that observed in mammals, albeit with some notable differences; for example, valine is orexigenic in fish, but not in mammals (Fig. 3). In fish, amino acid-sensing Journal of Experimental Biology (2024) 227, jeb247410. doi:10.1242/jeb.247410 mechanisms are predominantly activated by leucine, as observed in rainbow trout (Comesaña et al., 2018a,b, 2021b) and Atlantic salmon (Comesaña et al., 2021a). Valine has a limited effect on hypothalamic amino acid-sensing mechanisms in rainbow trout, but it exerts stronger effects in other brain areas, such as the telencephalon (Comesaña et al., 2018a,b, 2022). The mechanisms of amino acid sensing that have been characterized in the hypothalamus of these fish species are based on: (1) metabolism of BCAA, (2) metabolism of glutamine, (3) taste receptors, (4) amino acid carriers, (5) mTOR signalling pathway and (6) Gcn2 kinase. Each of these mechanisms were first characterised in mammals (although the specific mechanisms may differ in fish) and are discussed in more detail below. Metabolism of BCAA The metabolic breakdown of the three BCAAs involves the enzymes branched-chain aminotransferase (BCAT) and branchedchain α-keto acid dehydrogenase complex (BCKDH) (AdevaAndany et al., 2017). In mammals, food intake is reduced following increases in BCKDH activity or central administration of the transamination product of leucine by BCAT (Blouet et al., 2009), whereas deletion of BCAT reverses the effect (Purpera et al., 2012). In the hypothalamus of rainbow trout, this amino acid-sensing mechanism is activated by leucine through Bckdh (Comesaña et al., 2018a,b, 2021b). Metabolism of glutamine Amino acid levels can also be sensed through metabolism of glutamine, as BCAA metabolism and the glutamine–glutamate cycle are linked. BCAAs are precursors of glutamine, because the BCAT enzyme produces glutamate which, in turn, is converted to glutamine by the enzyme glutamine synthetase (GLS) (Adeva-Andany et al., 2017). In mammals, leucine stimulates the activity of the enzyme glutamate dehydrogenase (GDH) which produces α-ketoglutarate (a substrate for the Krebs cycle) from glutamate (Jewell and Guan, 2013). Thus, increased levels of leucine result in enhanced metabolism of glutamine and glutamate, and ATP production. In rainbow trout, leucine fails to stimulate Gdh activity in the hypothalamus (Comesaña et al., 2018a), but this mechanism is activated through increased Gls (Fig. 3; Comesaña et al., 2018a,b, 2021b). In addition, exposure to leucine decreases glutamate levels in zebrafish brain, supporting the link between leucine and the degradation of glutamate by Gdh (da Silva Lemos et al., 2022). Taste receptors In mammals, three receptors belonging to the taste receptor type 1 family (T1Rr1, T1R2 and T1R3) detect sweet and umami tastes. These receptors are also present in the mammalian hypothalamus, where they contribute to amino acid sensing. The receptors heterodimerise in the presence of glucose (T1R2–T1R3) and amino acids (T1R1–T1R3), triggering a cascade of second messengers that depolarizes the membrane (Fig. 3; Behrens and Meyerhof, 2016). In fish, unlike in mammals, amino acids can be detected by both heterodimers (Morais, 2017), and an increase in leucine levels increases the levels of t1r2 and t1r3 mRNA in the hypothalamus of rainbow trout (Comesaña et al., 2018a,b). Amino acid carriers Amino acid carriers provide an additional mechanism for sensing the levels of amino acids. Among all amino acid carriers, two are particularly noteworthy in this regard, and both fall within the solute carrier (SLC) superfamily; they are L-type amino acid transporter 1 7 Journal of Experimental Biology REVIEW REVIEW Journal of Experimental Biology (2024) 227, jeb247410. doi:10.1242/jeb.247410 Leu T1r1 T1r2 Leu Leu T1r3 Snat Lat1 2 3 4 Gln 2 1 Leu Gls Leu Bcat Sesn2 Gcn2 6 5 Glu KIC Bckdh mTOR Elf2D Plc/IP3 pathway Bckdk ER Ca2+ Acetyl-CoA Selective gene expression Ca2+ Ca2+ Nucleus ADP ATP K+ATP channel Respiratory chain NADH, FADH2 Krebs cycle Ca2+ ATP K+ Mitochondria Vdcc Ca2+ Vm (LAT1) and sodium-dependent neutral amino acid transporter 2 (SNAT2; Fig. 3). LAT1 and SNAT2 are present in the blood–brain barrier (see Glossary). They are key regulators of leucine intracellular concentration and are required for the activation of mTOR signalling (Hundal and Taylor, 2009; Dodd and Tee, 2012; Taylor, 2014). In fish hypothalamus, leucine upregulates snat2 expression in rainbow trout (Comesaña et al., 2018a,b) and both lat1 and snat2 in Atlantic salmon (Comesaña et al., 2021a), suggesting that these two amino acid carriers might act as independent amino acid-sensing mechanisms. mTOR signalling The mTOR signalling pathway in mammals is well known to be activated by nutrients, especially by leucine (Dodd and Tee, 2012; Yue et al., 2022), although the mechanism of action is unclear. Several studies have evaluated the role of mTOR signalling in amino acid sensing in the fish brain. For example, in rainbow trout hypothalamus, this mechanism is activated by leucine. In rainbow trout, ICV and IP treatment with leucine upregulates abundance of mtor mRNA and the phosphorylation status of the protein mTOR, i.e. the percentage of the protein in the phosphorylated (active) form (Comesaña et al., 2018a,b). Additionally, valine is also detected by this mechanism in the hypothalamus of rainbow trout (Comesaña et al., 2022), whereas in cultured brain cells of Chinese perch, leucine (but not valine or isoleucine) activates the downstream mTOR protein S6 (Chen et al., 2021). However, it has been suggested that the presence of insulin is also necessary for the activation of mTOR by leucine (Ahmad et al., 2021). In fact, in vitro incubation of rainbow trout hypothalamus with different concentrations of leucine in the absence of insulin does not affect mTOR or its downstream proteins but does increase levels of mRNA encoding the mTOR inhibitor Sesn2 (Comesaña et al., 2021b). The mTOR signalling pathway is also considered as an integrative pathway, linking nutrient-sensing information with the expression of neuropeptides involved in the regulation of feed intake (Soengas, 2021), as discussed below. Gcn2 kinase The amino acid-sensing mechanism that depends on GCN2 kinase (Fig. 3) is mostly activated under conditions of amino acid deficiency. When levels of circulating amino acids decline, tRNAs are no longer ‘charged’ (i.e. linked to an amino acid); uncharged tRNAs interact with and activate GCN2 kinase, which phosphorylates eukaryotic initiation factor 2α (eIF2α), thereby 8 Journal of Experimental Biology Fig. 3. Schematic summary of processes involved in amino acid sensing in the fish hypothalamus. A rise in circulating levels of leucine is sensed through different mechanisms. (1) Metabolic breakdown of leucine via Bcat and Bckdh leads to the production of acetyl-CoA and succinyl-CoA and subsequently ATP. (2) Production of glutamine. (3) Increased expression of T1r2 and T1r3. (4) Enhanced production of Lat1 and Snat2 carriers. (5) Activation of mTOR. (6) Activation of eIF2α. For definition of all symbols, see list of abbreviations. inhibiting it, and resulting in the selective control of translation (Battu et al., 2017). In fish, this mechanism has been studied in peripheral tissues with different amino acid deficiencies (SkibaCassy et al., 2016; Wang et al., 2016; Miao et al., 2021; Dou et al., 2023). The abundance of phosphorylated EIf2α protein increases with lysine deprivation in cultured brain cells of Chinese perch (Zou et al., 2022). However, this mechanism of amino acid sensing is apparently also responsive to leucine abundance because abundance of eIf2a mRNA increases in the hypothalamus of rainbow trout cultured with leucine in vitro (Comesaña et al., 2021b). Impact on feeding and metabolism Like diets that are high in carbohydrate or lipid, diets with elevated protein levels or higher levels of leucine also lead to a reduction in feed intake in fish (Table 1). This effect involves concomitant changes in npy, agrp and pomc gene expression. Neither valine nor isoleucine affect food intake in mammals, where leucine is the only BCAA involved in food intake regulation (Lueders et al., 2022). In fish, similar results are observed with isoleucine: ICV treatment of Chinese perch with isoleucine has no effect on food intake (Chen et al., 2021). However, in contrast to mammals, ICV administration of valine in fish produces an orexigenic response but without changes in neuropeptides (Table 1). Interestingly, ICV treatment with lysine or methionine in Chinese perch also elicits increased feed intake (Zou et al., 2022) Apart from detecting the presence of nutrients to allow the regulation of feed intake, the fish hypothalamus also uses this information to regulate peripheral metabolism, thus maintaining energy homeostasis (Soengas et al., 2018). In mammals, amino acid levels and glucose metabolism are closely related – the hypothalamic detection of leucine also modulates hepatic metabolism, with the effect of reducing glucose production (Su et al., 2012; Arrieta-Cruz and Gutiérrez-Juárez, 2016). This effect has not yet been demonstrated in fish, but some studies have shown that central detection of amino acids in fish has peripheral effects. Thus, in Chinese perch, levels of preproghrelin in the intestine and leptin in the liver change after ICV injection of leucine, valine or isoleucine (Chen et al., 2021). In addition, in the same species, ICV injection of valine acts as a nutritional signal in the brain to modulate peripheral metabolism, attenuating protein degradation (Wang et al., 2020). Integration of nutrient-sensing systems Once nutrient-sensing systems are activated/inhibited, the relevant information is integrated in the hypothalamus through changes in the levels of agrp/npy and pomc/cart mRNA, which all encode neuropeptides involved in the regulation of feed intake (Fig. 4). This occurs through changes in signalling pathways, causing the activation/inhibition of transcription factors that ultimately regulate feed intake. The relevant mechanisms of integration are well understood in mammals (for reviews, see Blouet and Schwartz, 2010; Morton et al., 2014). Below, we discuss the current state of knowledge around the hypothalamic integration of nutrient-sensing information in fish. Signalling pathways The AMP-activated protein kinase pathway In mammals, one of the hypothalamic signalling pathways that is sensitive to changes in nutrient-sensing systems is the AMP-activated protein kinase (AMPK) pathway. The levels of hypothalamic AMPK and the phosphorylation status of the protein decrease as the levels of glucose, fatty acids or amino acids increase (Cai et al., 2007; Beall et al., 2012; Fromentin et al., 2012; Oh et al., 2016). Ampk is present Journal of Experimental Biology (2024) 227, jeb247410. doi:10.1242/jeb.247410 in fish brain (Soengas, 2021), but few studies have addressed changes under conditions relating to the control of feed intake. The results of those that have are mostly in agreement with the mammalian model (Fig. 4). For example, feed deprivation results in increased levels and phosphorylation status of the protein Ampkα in the hypothalamus of rainbow trout (Conde-Sieira et al., 2019) (although note that this is not seen in the brain of channel catfish, Ictalurus punctatus; Abernathy et al., 2019). Furthermore, when rainbow trout are fed a lipid-enriched diet (Librán-Pérez et al., 2015a), the phosphorylation of Ampkα in the hypothalamus decreases. Similar changes are seen in fish with hyperglycaemia or those fed a diet high in carbohydrates (Nipu et al., 2022; Kamalam et al., 2012; Xu et al., 2017). In rainbow trout, the phosphorylation of Ampkα is decreased in response to higher levels of nutrients such as oleate (Velasco et al., 2016a, 2017b; Blanco et al., 2020), octanoate (Velasco et al., 2017b), glucose (Otero-Rodiño et al., 2017) or β-hydroxybutyrate (Comesaña et al., 2019). In contrast to the mammalian model, the phosphorylation of Ampkα is not increased in rainbow trout hypothalamus when the levels of leucine are increased (Comesaña et al., 2018a,b, 2020, 2021). The involvement of Ampk in the integration of nutrient sensing is further supported by the fact that the phosphorylation of Ampkα decreases in rainbow trout hypothalamus following treatments that suppress appetite (i.e. treatments that mimic the activation of nutrientsensing pathways; Velasco et al., 2016b, 2017a, 2019, 2020, 2021; Blanco et al., 2020). In contrast, it should be noted that Siberian sturgeon treated with the anorexigenic hormone adiponectin show enhanced levels of Ampkα2 (Tang et al., 2022). There are several different isoforms of Ampkα, and it seems that Ampkα2 is involved in regulation of feed intake, whereas Ampkα1 appears to modulate peripheral metabolism to maintain homeostasis, as observed in rainbow trout (Conde-Sieira et al., 2019, 2020). Finally, it is known that there is an interaction between hormones and the integration of nutrient-sensing pathways through Ampk, because the presence of oleate counteracts the stimulatory effect of ghrelin on Ampk in rainbow trout hypothalamus (Velasco et al., 2016a). The mTOR signalling pathway As discussed above, mTOR is thought to play an important role in integrating nutrient-sensing pathways. In fish, mTOR is involved in regulation of feed intake. In zebrafish, feed deprivation results in a decrease in mTOR levels (Craig and Moon, 2011), whereas mTOR levels and/or phosphorylation status increase under the anorectic (i.e. appetite-suppressing) conditions elicited by the presence of nutrients, as demonstrated in rainbow trout (Velasco et al., 2017b; Comesaña et al., 2018a,b; Blanco et al., 2020). Accordingly, ICV treatment with the appetite-stimulating amino acid valine in rainbow trout results in decreased levels of mTOR (Comesaña et al., 2022). The response of mTOR to changes in nutrient levels is also supported by changes in its downstream proteins. For example, in Chinese perch, the levels of phosphorylated S6 increase after ICV treatment with the anorexigenic amino acid leucine, whereas a decrease occurs after treatment with the orexigenic amino acid valine (Chen et al., 2021). Interestingly, these changes are abolished in the additional presence of rapamycin, an inhibitor of mTOR (Chen et al., 2021). It should be noted that the involvement of mTOR in the integration of nutrient sensing might depend on the different species assessed, as well as on the specific amino acid involved. For example, in rainbow trout, leucine has an anorectic effect; however, in vitro, rainbow trout hypothalamus does not show any response to leucine in terms of the levels and phosphorylation status of mTOR or any effects on downstream proteins (Comesaña 9 Journal of Experimental Biology REVIEW REVIEW Journal of Experimental Biology (2024) 227, jeb247410. doi:10.1242/jeb.247410 Assimilation and storage Feeding Hormones Nutrients e.g. Ghrl, Cck, Pyy, Glp-1, Insulin, Leptin Glucose Amino acids Fatty acids Ingestion and rejection Hormone receptors Nutrient sensing mTOR Akt Other signals? Ampk Foraging and procurement Nucleus of orexigenic neuron Creb Bsx Nucleus of anorexigenic neuron Foxo1 Npy/Agrp Bsx Second order neurons (NPO, NAT, NLTI, NLTd, other areas?) Pomc/Cart Hypothalamic NLTv Feed intake et al., 2021a,b). This suggests that the anorectic effect of leucine in rainbow trout is independent of mTOR signalling in the hypothalamus. In contrast, a comparable treatment with the orexigenic amino acid valine does result in changes in mTOR and downstream proteins (Comesaña et al., 2022). Additional studies support a role for hypothalamic mTOR in feed-intake regulation in fish, supporting its role as an integrator of nutritional signals. Multiple treatments that induce anorectic conditions in fish cause an increase in mTOR levels and/or phosphorylation status (Penney and Volkoff, 2014; Librán-Pérez et al., 2015a,b; Dai et al., 2018; Velasco et al., 2017a, 2018, 2019, 2021; Blanco et al., 2020). Furthermore, hypothalamic mTOR activation modulates the abundance of pomc and npy mRNA in Japanese sea bass (Liang et al., 2019). The protein kinase B (Akt) signalling pathway In the mammalian hypothalamus, Akt levels and phosphorylation status are increased in the presence of higher levels of nutrients (Hu et al., 2016; Park et al., 2011). Current evidence suggests that Akt is also involved in the hypothalamic mechanisms that regulate feed intake in fish (Fig. 4). For example, exposure to various nutrients in vivo and in vitro activates Akt signalling through increased phosphorylation of the Akt protein (Velasco et al., 2017b; OteroRodiño et al., 2017; Comesaña et al., 2018a; Blanco et al., 2020). Furthermore, a comparable increase in Akt protein levels occurs in the brains of fish fed diets enriched in specific nutrients, including carbohydrates (Dai et al., 2014; Jin et al., 2014; Jörgens et al., 2015) or lipids (Librán-Pérez et al., 2015a,b; Dai et al., 2018; Xu et al., 2019). Akt activation also occurs under other conditions that elicit anorectic responses (Gong et al., 2016; Velasco et al., 2016b, 2017a, 2020, 2021; Blanco et al., 2020). The involvement of hypothalamic Akt in regulation of feed intake is also supported by the fact that an opposing response (decreased phosphorylation status) is observed under orexigenic conditions (Velasco et al., 2017a). In mammals, the activation of Akt signalling in the hypothalamus results in changes in fatty acid metabolism through the activation of the transcription factor SREBP1c and its targets ATP citrate lyase (ACLY) and FAS (Kim et al., 2007). Accordingly, in the rainbow trout hypothalamus, enhanced phosphorylation of Akt occurs in parallel with increased levels of acly, fas and srebp1c mRNA (Velasco et al., 2016b), thus indirectly supporting the involvement of Akt in signalling related to nutrient sensing. Transcription factors Brain homeobox transcription factor In mammals, brain homeobox transcription factor (BSX) is one of several transcription factors that are activated in response to the signalling pathways discussed above. It interacts with cAMP responseelement binding protein (CREB; see below) in the mammalian hypothalamus, leading to an increase in mRNA encoding BSX, 10 Journal of Experimental Biology Fig. 4. Schematic summary of processes involved in the integration of nutrient-sensing information in the fish hypothalamus. After processes involved in feed procurement, ingestion and assimilation (orange boxes), nutrient-sensing systems (blue boxes) are activated in parallel with changes in hormone levels ( pink boxes). This results in changes in signalling pathways (green boxes), including Ampk inhibition, and activation of Akt and mTOR. Changes in these signalling pathways alters the phosphorylation status of the transcription factors Creb, Foxo1 and Bsx, leading to inhibition of feed intake ( purple box) via changes in the expression of neuropeptides in orexigenic (Npy/Agrp) and anorexigenic (Pomc/Cart) neurons of the hypothalamic NLTv. Synaptic connections (grey circles) between orexigenic and anorexigenic neurons as well as with other second-order neurons modulate the responses. Together, this integration results in the homeostatic control of feed intake. For definition of all symbols, see list of abbreviations. NPY and AgRP (Fig. 4; Nogueiras et al., 2008; Varela et al., 2011; Lee et al., 2016). BSX levels are reduced under anorectic conditions and increase under orexigenic conditions (Nogueiras et al., 2008; Lage et al., 2010). Only a few studies in fish have evaluated changes in Bsx that could relate to the regulation of feed intake. Thus, we know that feed deprivation in goldfish increases bsx mRNA levels in the hypothalamus (Vinnicombe and Volkoff, 2022). Accordingly, in rainbow trout, exposure to oleate (Conde-Sieira et al., 2018), leucine (Comesaña et al., 2021a,b) or glucose (Conde-Sieira et al., 2018; Blanco et al., 2020) (all of which reduce feed intake) results in decreased levels of Bsx. However, ICV treatment with the orexigenic amino acid valine also results in a decrease in levels of Bsx protein in rainbow trout (Comesaña et al., 2022). The effects of glucose on Bsx in the rainbow trout hypothalamus disappear after insulin treatment (Blanco et al., 2020), suggesting that there is an interaction between this nutrient and hormone that has an impact on integrative hypothalamic mechanisms. Other conditions that are known to induce an anorexigenic response in rainbow trout also decrease the levels of Bsx protein (Velasco et al., 2019, 2020, 2021). However, in goldfish, treatment with the anorexigenic hormone Cck does not alter levels of bsx mRNA (Vinnicombe and Volkoff, 2022). cAMP response-element binding protein CREB is another transcription factor that is thought to be involved in the connection between brain metabolism and the expression of neuropeptides that regulate appetite in mammals. A decrease in CREB levels in the mammalian brain induces a decrease in the abundance of Npy and Agrp mRNA, resulting in decreased food intake (Fig. 4; Belgardt et al., 2009; Varela et al., 2011; Blanco de Morentín et al., 2011). In fish, the available information regarding the possible involvement of Creb in regulation of feed intake is scarce and is mostly restricted to rainbow trout. In this species, Creb phosphorylation decreases in response to raised levels of oleate (Velasco et al., 2017b; Conde-Sieira et al., 2018), octanoate (Velasco et al., 2017b) and glucose (Conde-Sieira et al., 2018; Otero-Rodiño et al., 2019b). In response to leucine, the phosphorylation of Creb is decreased in rainbow trout in vivo after ICV (Comesaña et al., 2018a) or IP (Comesaña et al., 2018b) treatment, but no changes occur in response to leucine under in vitro conditions (Comesaña et al., 2021a,b). Interestingly, ICV treatment of rainbow trout with valine also fails to elicit a Creb response (Comesaña et al., 2022), as does supplementation of feed with methionine in zebrafish (Pisera-Fuster et al., 2021). The presence of an inhibitor of Creb blocks the response of Creb to fatty acids in rainbow trout hypothalamus (Velasco et al., 2017b), indirectly supporting its role in regulation of feed intake. The changes in Creb that occur in response to changes in nutrient levels are comparable to those observed under other anorectic conditions in rainbow trout, such as treatment with the anorexigenic hormones Cck or Glp-1 (Velasco et al., 2019). Moreover, in zebrafish, increased levels of Creb also occur under the orexigenic conditions elicited by feed deprivation (Craig and Moon, 2011). Forkhead box O1 Forkhead box O1 (FoxO1) is likely to be involved in the hypothalamic integration of nutrient-sensing pathways to regulate feed intake in mammals (Gross et al., 2009). In mammals, conditions that increase the levels of FoxO1 protein cause an increase in the expression of Agrp mRNA while decreasing the levels of Pomc mRNA; changes that lead to a decrease in food intake (Belgardt et al., 2009; Blanco de Morentin et al., 2011). In general, in fish, increased levels of nutrients result in increased abundance and phosphorylation of the protein Foxo1, as observed in rainbow trout Journal of Experimental Biology (2024) 227, jeb247410. doi:10.1242/jeb.247410 in response to increased levels of oleate (Conde-Sieira et al., 2018; Blanco et al., 2020), octanoate (Velasco et al., 2017b) and glucose (Conde-Sieira et al., 2018; Blanco et al., 2020). The lack of response of rainbow trout hypothalamus to oleate in the presence of a Foxo1 inhibitor supports the specificity of this response (Velasco et al., 2017b). There is also evidence for an interaction between nutrient levels and hormones in the dynamics of Foxo1 in the hypothalamic response: in rainbow trout, the presence of insulin counteracts the effects of oleate on the abundance of foxo1 mRNA (Blanco et al., 2020). In contrast to its response to glucose or fatty acids, hypothalamic Foxo1 does not appear to respond to changes in the levels of amino acids in fish, as demonstrated in rainbow trout (Comesaña et al., 2018a,b, 2020, 2021a,b, 2022). A range of anorectic conditions other than those produced by increased nutrient levels also result in increased levels of Foxo1 protein in rainbow trout (Velasco et al., 2016b, 2017a, 2019, 2020, 2021; Blanco et al., 2020). Conclusions and future directions In this Review, we present an overview of how the fish hypothalamus integrates metabolic and endocrine information to elicit changes in feed intake and peripheral energy metabolism. The underlying mechanisms are similar in some respects to those known in mammals, but are not identical (Delgado et al., 2017; Soengas et al., 2018). The relevant metabolic effects result from the activation/inhibition of nutrient-sensing systems. Glucosensing mechanisms in the fish hypothalamus involve a complex interplay of multiple systems, including the well known Gck–Glut2 pathway, Sglt-1, sweet taste receptors, Lxr and potential mitochondria-based mechanisms. Together, these mechanisms enable the hypothalamus to finely regulate responses to changes in glucose levels. Glucosensing in the hypothalamus influences feeding behaviour, the expression of appetite-related neuropeptides and metabolic responses in fish. Future research in this area should focus on: (1) investigating changes in the discharge frequency of neurons in response to circulating glucose concentrations; (2) the precise roles of the glucosensing systems not dependent on Gck– Glut2; (3) the importance of astrocytes and tanycytes; and (4) investigating how chronic changes in glucose levels, induced through diet or environmental conditions, impact overall metabolic health and energy balance in fish. Compared with glucose-sensing pathways, fish and mammals are more divergent in terms of their fatty acid-sensing mechanisms. Unlike that of mammals, the fish hypothalamus responds not only to LCFAs but also to MCFAs, reflecting the unique metabolic adaptations in fish. Mechanisms involving malonyl-CoA, enzymes such as Cpt-1, Fas and Acly, as well as the fatty acid translocase (Fat/cd36), have been identified as crucial components of fatty acid sensing in fish. Moreover, the impact of fatty acid sensing on feeding regulation and metabolic processes in fish is evident. Further research is needed to delve deeper into the specific signaling pathways involved, particularly: (1) the identification and characterization of Ffar1, Gpr84 and Gpr119; (2) investigating the crosstalk between central (hypothalamus) and peripheral (liver, Brockman bodies) fatty acidsensing mechanisms; and (3) exploring species-specific adaptations and responses to diverse types of fatty acids, considering the diversity in fish physiology and ecology. Amino acid sensing has only recently been investigated in fish hypothalamus. However, leucine has already emerged as a crucial amino acid influencing regulation of feed intake in both mammals and fish, albeit with species-specific responses. The effects of amino acids such as valine, lysine and methionine on feed intake in fish also differ 11 Journal of Experimental Biology REVIEW from the responses in mammals. Understanding these differences will shed light on the diverse metabolic adaptations across species. Because most of our information on amino acid sensing in fish is restricted to a few species, it is essential to enhance our understanding of the universality or specificity of the relevant mechanisms in fish species other than salmonids. Furthermore, the roles of amino acid carriers, such as Lat1 and Snat2, in the fish hypothalamus need further exploration. Considering the carnivorous nature of many fish species, understanding their amino acid sensing will have practical applications in optimizing dietary formulations for improved growth and health in farmed fish. The hypothalamic integration of neuroendocrine signalling and nutrient sensing in the regulation of feed intake is a complex and intricate process, of which our current understanding is limited. We have identified some of the signalling pathways and transcription factors involved in this integration, but changes in feed intake stemming from alterations in neuropeptide expression are also the result of a complex interplay between nutrient and hormonal signal transduction. This interaction remains largely unexplored; for example, in mammals we have only limited evidence pertaining to the interactive effects of hormones such as leptin or ghrelin on fatty acid sensing (López et al., 2007; Blanco de Morentin et al., 2011; Lockie et al., 2019). In fish, studies conducted in rainbow trout have demonstrated the existence of interactive effects between nutrient-sensing mechanisms and hormones such as ghrelin (Velasco et al., 2016a) and insulin (Blanco et al., 2020). In conclusion, what we know of glucosensing, fatty acid sensing and amino acid sensing mechanisms in the fish hypothalamus underscores the remarkable adaptability of fish to varying nutritional conditions. These diverse sensory pathways – involving well-established systems such as Gck–Glut2, as well as newly assessed components, such as fatty acid translocase and amino acid carriers – play pivotal roles in regulating feeding behaviour and metabolic responses. The complex integration of neuroendocrine signalling, transcription factors and nutrient sensing in the hypothalamus highlights the need for further research to unravel the species-specific nuances. As our understanding deepens, addressing the long-term effects of altered sensing mechanisms on energy homeostasis in fish becomes essential. This holistic perspective not only enhances our knowledge of fundamental physiological processes but also holds promise for optimizing dietary formulations and promoting sustainable and efficient aquaculture practices. Competing interests The authors declare no competing or financial interests. Funding The authors acknowledge the support of research grants from the Agencia Estatal de Investigació n and European Regional Development Fund (PID2022–136288OBC31/AEI/10.13039/501100011033/FEDER,UE) and Xunta de Galicia (Axudas para a consolidació n e estruturació n de unidades de investigació n competitivas e outras acció ns de fomento nas universidades do SUG, ED431B 2022/01) to J.L.S. A.M.B. was recipient of a postdoctoral fellowship (Program Ramó n y Cajal) from Ministerio de Ciencia e Innovació n y Universidades (MICIU) (RYC2022-037124-I). References Abernathy, O., Kostner, D., Buer, P., Dougherty, M., Schmidtberger, A., Spainhour, R., Leiker, A., Vides, M., Teel, B. and Kobayashi, Y. (2019). Expression of messenger RNA encoding two cellular metabolic regulators, AMPactivated protein kinase (AMPK) and O-GlcNAc transferase (OGT), in channel catfish: their tissue distribution and relationship with changes in food intake. Comp. Biochem. Physiol. A 235, 12-21. doi:10.1016/j.cbpa.2019.04.023 Adeva-Andany, M. M., Ló pez-Maside, L., Donapetry-Garcı́a, C., Ferná ndezFerná ndez, C. and Sixto-Leal, C. (2017). Enzymes involved in branched-chain amino acid metabolism in humans. Amino Acids 49, 1005-1028. doi:10.1007/ s00726-017-2412-7 Journal of Experimental Biology (2024) 227, jeb247410. doi:10.1242/jeb.247410 Aguilar, A. J., Conde-Sieira, M., Ló pez-Patino, ̃ M. A., Mı́guez, J. M. and Soengas, J. L. (2011). In vitro leptin treatment of rainbow trout hypothalamus and hindbrain affects glucosensing and gene expression of neuropeptides involved in food intake regulation. Peptides 32, 232-240. doi:10.1016/j.peptides.2010.11.007 Ahmad, I., Ahmed, I., Fatma, S. and Peres, H. (2021). Role of branched-chain amino acids on growth, physiology and metabolism of different fish species: A review. Aquaculture Nutr 27, 1270-1289. doi:10.1111/anu.13267 Anthonisen, E. H., Berven, L., Holm, S., Nygård, M., Nebb, H. I. and GrØnningWang, L. M. (2010). Nuclear receptor liver X receptor is O-GlcNAc-modified in response to glucose. J. Biol. Chem. 285, 1607-1615. doi:10.1074/jbc.m109. 082685 Arrieta-Cruz, I. and Gutié rrez-Juá rez, R. (2016). The role of circulating amino acids in the hypothalamic regulation of liver glucose metabolism. Advan. Nutr. 7, 790S-797S. doi:10.3945/an.115.011171 Babaei, S., Sá ez, A., Caballero-Solares, A., Ferná ndez, F., Baanante, I. V. and Metó n, I. (2017). Effect of dietary macronutrients on the expression of cholecystokinin, leptin, ghrelin and neuropeptide Y in gilthead sea bream (Sparus aurata). Gen. Comp. Endocrinol. 240, 121-128. doi:10.1016/j.ygcen. 2016.10.003 Balasubramanian, M. N., Panserat, S., Dupont-Nivet, M., Quillet, E., Montfort, J., Le Cam, A., Medale, F., Kaushik, S. J. and Geurden, I. (2016). Molecular pathways associated with the nutritional programming of plant-based diet acceptance in rainbow trout following an early feeding exposure. BMC Genomics 17, 1-20. doi:10.1186/s12864-016-2804-1 Basto-Silva, C., Enes, P., Oliva-Teles, A., Balbuena-Pecino, S., Navarro, I., Capilla, E. and Guerreiro, I. (2021). Dietary protein source and protein/ carbohydrate ratio affects appetite regulation-related genes. Aquaculture 533, 736142. doi:10.1016/j.aquaculture.2020.736142 Basto-Silva, C., Couto, A., Rodrigues, J., Oliva-Teles, A., Navarro, I., Kaiya, H., Capilla, E. and Guerreiro, I. (2022). Feeding frequency and dietary protein/ carbohydrate ratio affect feed intake and appetite regulation-related genes expression in gilthead seabream (Sparus aurata). Comp. Biochem. Physiol. A 267, 111168. doi:10.1016/j.cbpa.2022.111168 Battu, S., Minhas, G., Mishra, A. and Khan, N. (2017). Amino acid sensing via general control nonderepressible-2 kinase and immunological programming. Front. Immunol. 8, 1719. doi:10.3389/fimmu.2017.01719 Beall, C., Hamilton, D. L., Gallagher, J., Logie, L., Wright, K., Soutar, M. P., Dadak, S., Ashford, F. B., Haythorne, E., Du, Q. et al. (2012). Mouse hypothalamic GT1-7 cells demonstrate AMPK-dependent intrinsic glucosesensing behaviour. Diabetologia 55, 2432-2444. doi:10.1007/s00125-012-2617-y Behrens, M. and Meyerhof, W. (2016). The vertebrate gustatory system. In Flavour, From Food to Perception (ed. E. Guichard, C. Salles, M. Morzel and A. M. Le Bon), pp. 57-78. Oxford, UK: Wiley Blackwell. Belgardt, B. F., Okamura, T. and Brü ning, J. C. (2009). Hormone and glucose signalling in POMC and AgRP neurons. J. Physiol. 587, 5305-5314. doi:10.1113/ jphysiol.2009.179192 Benoit, S. C., Kemp, C. J., Elias, C. F., Abplanalp, W., Herman, J. P., Migrenne, S., Lefevre, A.-L., Cruciani-Guglielmacci, C., Magnan, C., Yu, F. et al. (2009). Palmitic acid mediates hypothalamic insulin resistance by altering PKC-θ subcellular localization in rodents. J. Clin. Invest. 119, 2577-2589. doi:10.1172/ JCI36714 Blanco, A. M., Bertucci, J. I., Soengas, J. L. and Unniappan, S. (2020). In vitro insulin treatment reverses changes elicited by nutrients in cellular metabolic processes that regulate food intake in fish. J. Exp. Biol. 223, jeb213454. doi:10. 1242/jeb.213454 Blanco de Morentin, P. B., Gonzá lez, C. R., Saha, A. K., Martins, L., Dié guez, C., Vidal-Puig, A., Tena-Sempere, M. and Ló pez, M. (2011). Hypothalamic AMPactivated protein kinase as a mediator of whole body energy balance. Rev. Endocrinol. Metab. Dis. 12, 127-140. doi:10.1007/s11154-011-9165-5 Blouet, C. and Schwartz, G. J. (2010). Hypothalamic nutrient sensing in the control of energy homeostasis. Behav. Brain Res. 209, 1-12. doi:10.1016/j.bbr. 2009.12.024 Blouet, C., Jo, Y. H., Li, X. and Schwartz, G. J. (2009). Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamusbrainstem circuit. J. Neurosci. 29, 8302-8311. doi:10.1523/JNEUROSCI.1668-09. 2009 Bodas, D. S., Maduskar, A., Kaniganti, T., Wakhloo, D., Balasubramanian, A., Subhedar, N. and Ghose, A. (2023). Convergent energy state-dependent antagonistic signaling by cocaine- and amphetamine-regulated transcript (CART) and neuropeptide Y (NPY) modulates the plasticity of forebrain neurons to regulated feeding in zebrafish. J. Neurosci. 43, 1089-1110. doi:10.1523/ JNEUROSCI.2426-21.2022 Bonacic, K., Campoverde, C., Gó mez-Arboné s, J., Gisbert, E., Estevez, A. and Morais, S. (2016). Dietary fatty acid composition affects food intake and gut–brain satiety signaling in Senegalese sole (Solea senegalensis, Kaup 1858) larvae and post-larvae. Gen. Comp. Endocrinol. 228, 79-94. doi:10.1016/j.ygcen.2016. 02.002 Burnstock, G. (1959). The innervation of the gut of the brown trout (Salmo trutta). J. Cell Sci. S3-100, 199-220. doi:10.1242/jcs.s3-100.50.199 12 Journal of Experimental Biology REVIEW Cai, F., Gyulkhandanyan, A. V., Wheeler, M. B. and Belsham, D. D. (2007). Glucose regulates AMP-activated protein kinase activity and gene expression in clonal, hypothalamic neurons expressing proopiomelanocortin: additive effects of leptin and insulin. J. Endocrinol. 192, 605-614. doi:10.1677/JOE-06-0080 Capilla, E., Mé dale, F., Navarro, I., Panserat, S., Vachot, C., Kaushik, S. and Gutié rrez, J. (2003). Muscle insulin binding and plasma levels in relation to liver glucokinase activity, glucose metabolism and dietary carbohydrates in rainbow trout. Reg. Peptides 110, 123-132. doi:10.1016/S0167-0115(02)00212-4 Chen, K., Zhang, Z., Li, J., Xie, S., Shi, L.-J., He, Y.-H., Liang, X.-F., Zhu, Q.-S. and He, S. (2021). Different regulation of branched-chain amino acid on food intake by TOR signaling in Chinese perch (Siniperca chuatsi). Aquaculture 530, 735792. doi:10.1016/j.aquaculture.2020.735792 Chen, H.-T., Li, L.-L., Wang, L.-H., Cheng, D.-H., Ma, H., Sun, M.-J., Yang, Y.-O. and Yuan, X.-C. (2022). Sweet taste receptors are the potential mediator involved in appetite regulation of grass carp in response to high digestible carbohydrates intake. Aquaculture Rep. 27, 101386. doi:10.1016/j.aqrep.2022.101386 Comesana, ̃ S., Velasco, C., Ceinos, R. M., Ló pez Patino, ̃ M. A., Mı́guez, J. M., Morais, S. and Soengas, J. L. (2018a). Evidence for the presence in rainbow trout brain of amino acid-sensing systems involved in the control of food intake. Am. J. Physiol. Reg. Integr. Comp. Physiol. 314, R201-R215. doi:10.1152/ ajpregu.00283.2017 Comesaña, S., Velasco, C., Conde-Sieira, M., Mı́guez, J. M., Soengas, J. L. and Morais, S. (2018b). Feeding stimulation ability and central effects of intraperitoneal treatment of L-leucine, L-valine, and L-proline on amino acid sensing systems in rainbow trout: implication in food intake control. Front. Physiol. 9, 1209. doi:10.3389/fphys.2018.01209 Comesaña, S., Velasco, C., Conde-Sieira, M., Otero-Rodiño, C., Mı́guez, J. M. and Soengas, J. L. (2019). Central treatment of ketone body in rainbow trout alters liver metabolism without apparently altering the regulation of food intake. Front. Physiol. 10, 1206. doi:10.3389/fphys.2019.01206 Comesana, ̃ S., Conde-Sieira, M., Velasco, C., Soengas, J. L. and Morais, S. (2020). Oral and pre-absorptive sensing of amino acids relates to hypothalamic control of food intake in rainbow trout. J. Exp. Biol. 223, jeb221721. doi:10.1242/ jeb.221721 Comesaña, S., Lai, F., Olderbakk Jordal, A. E., Verri, T., Espe, M., Soengas, J. L. and Rønnestad, I. (2021a). Amino acid carriers of the solute carrier families 7 (SLC7) and 38 (SLC38) are involved in leucine sensing in the brain of Atlantic salmon (Salmo salar). Front. Mar. Sci. 8, 1-16. doi:10.3389/fmars.2021.711508 Comesana, ̃ S., Velasco, C. and Soengas, J. L. (2021b). Leucine sensing in rainbow trout hypothalamus is direct but separate from mTOR signalling in the regulation of food intake. Aquaculture 543, 737009. doi:10.1016/j.aquaculture. 2021.737009 Comesaña, S., Chivite, M., Blanco, A. M., Alborja-Valado, M., Calo, J., CondeSieira, M. and Soengas, J. L. (2022). Involvement of mechanistic target of rapamycin (mTOR) in valine orexigenic effects in rainbow trout. Aquaculture Nutr. 2022, 7509382. doi:10.1155/2022/7509382 Conde-Sieira, M. and Soengas, J. L. (2017). Nutrient sensing systems in fish: impact on food intake regulation and energy homeostasis. Front. Neurosci. 10, 603. doi:10.3389/fnins.2016.00603 Conde-Sieira, M., Aguilar, A. J., Ló pez-Patiño, M. A., Mı́guez, J., Soengas, M. and L, J. (2010a). Stress alters food intake and glucosensing response in hypothalamus, hindbrain, liver, and Brockmann bodies of rainbow trout. Physiol. Behav. 101, 483-493. doi:10.1016/j.physbeh.2010.07.016 Conde-Sieira, M., Agulleiro, M. J., Aguilar, A. J., Mı́guez, J. M., Cerdá -Reverter, J. M. and Soengas, J. L. (2010b). Effect of different glycaemic conditions on gene expression of neuropeptides involved in control of food intake in rainbow trout; interaction with stress. J. Exp. Biol. 213, 3858-3865. doi:10.1242/jeb.048439 Conde-Sieira, M., Librá n-Pé rez, M., Ló pez Patiño, M. A., Mı́guez, J. M. and Soengas, J. L. (2011). CRF treatment induces a readjustment in glucosensing capacity in the hypothalamus and hindbrain of rainbow trout. J. Exp. Biol. 214, 3887-3894. doi:10.1242/jeb.061564 Conde-Sieira, M., Librá n-Pé rez, M., Ló pez Patino, ̃ M. A., Soengas, J. L. and Mı́guez, J. M. (2012). Melatonin treatment alters glucosensing capacity and mRNA expression levels of peptides related to food intake control in rainbow trout hypothalamus. Gen. Comp. Endocrinol. 178, 131-138. doi:10.1016/j.ygcen.2012. 04.011 Conde-Sieira, M., Alvarez, R., Ló pez-Patiño, M. A., Mı́guez, J. M., Flik, G. and Soengas, J. L. (2013). ACTH stimulated cortisol release from head kidney of rainbow trout is modulated by glucose concentration. J. Exp. Biol. 216, 554-567. doi:10.1242/jeb.076505 Conde-Sieira, M., Bonacic, K., Velasco, C., Valente, L. M. P., Morais, S. and Soengas, J. L. (2015). Hypothalamic fatty acid sensing in Senegalese sole (Solea senegalensis): response to long-chain saturated, monounsaturated, and polyunsaturated (n-3) fatty acids. Am. J. Physiol. Reg. Integ. Comp. Physiol. 309, R1521-R1531. doi:10.1152/ajpregu.00386.2015 Conde-Sieira, M., Ceinos, R. M., Velasco, C., Comesaña, S., Ló pez-Patiño, M. A., Mı́guez, J. M. and Soengas, J. L. (2018). Response of rainbow trout (Oncorhynchus mykiss) hypothalamus to glucose and oleate assessed through transcription factors BSX, ChREBP, CREB, and FoxO1. J. Comp. Physiol. A. 204, 893-904. doi:10.1007/s00359-018-1288-7 Journal of Experimental Biology (2024) 227, jeb247410. doi:10.1242/jeb.247410 Conde-Sieira, M., Capelli, V., Álvarez-Otero, R., Comesana, ̃ S., Linares-Pose, ̃ L., Velasco, C., Ló pez, M. and Soengas, J. L. (2019). Differential role of hypothalamic AMPKα isoforms in fish: an evolutive perspective. Mol. Neurobiol. 56, 5051-5066. doi:10.1007/s12035-018-1434-9 Conde-Sieira, M., Capelli, V., Álvarez-Otero, R., Dı́az-Rú a, A., Velasco, C., Comesaña, S., Ló pez, M. and Soengas, J. L. (2020). Hypothalamic AMPKα2 regulates liver energy metabolism in rainbow trout through vagal innervation. Am. J. Physiol. Reg. Integ. Comp. Physiol. 318, R122-R134. doi:10.1152/ AJPREGU.00264.2019 Craig, P. M. and Moon, T. W. (2011). Fasted zebrafish mimic genetic and physiological responses in mammals: a model for obesity and diabetes? Zebrafish 8, 109-117. doi:10.1089/zeb.2011.0702 Craig, P. M., Massarsky, A. and Moon, T. W. (2013). Understanding glucose uptake during methionine deprivation in incubated rainbow trout (Oncorhynchus mykiss) hepatocytes using a non-radioactive method. Comp. Biochem. Physiol. B 166, 23-29. doi:10.1016/j.cbpb.2013.06.005 da Silva Lemos, I., Wessler, L. B., Duarte, M. B., da Silva, G. L., Bernardo, H. T., Candiotto, G., Torres, C. A., Petronilho, F., Rico, E. P. and Streck, E. L. (2022). Exposure to leucine alters glutamate levels and leads to memory and social impairment in zebrafish. Metab. Brain Dis. 37, 2925-2935. doi:10.1007/s11011022-01070-w Dai, W., Panserat, S., Terrier, F., Seiliez, I. and Skiba-Cassy, S. (2014). Acute rapamycin treatment improved glucose tolerance through inhibition of hepatic gluconeogenesis in rainbow trout (Oncorhynchus mykiss). Am. J. Physiol. Reg. Integ. Comp. Physiol. 307, R1231-R1238. doi:10.1152/ajpregu.00166.2014 Dai, Y.-J., Jiang, G.-Z., Yuan, X.-Y. and Liu, W.-B. (2018). High-fat-diet-induced inflammation depresses the appetite of blunt snout bream (Megalobrama amblycephala) through the transcriptional regulation of leptin/mammalian target of rapamycin. Brit. J. Nutr. 120, 1422-1431. doi:10.1017/S000711451800288X De Backer, I., Hussain, S. S., Bloom, S. R. and Gardiner, J. V. (2016). Insights into the role of neuronal glucokinase. Am. J. Physiol. Endocrinol. Metab. 311, E42-E55. doi:10.1152/ajpendo.00034.2016 Delgado, M. J., Cerdá -Reverter, J. M. and Soengas, J. L. (2017). Hypothalamic integration of metabolic, endocrine, and circadian signals in fish: involvement in the control of food intake. Front. Neurosci. 11, 354. doi:10.3389/fnins.2017.00354 Dı́ez-Sampedro, A., Hirayama, B. A., Osswald, C., Gorboulev, V., Baumgarten, K., Volk, C., Wright, E. M. and Koepsell, H. (2003). A glucose sensor hiding in a family of transporters. Proc. Natl. Acad. Sci. USA 100, 11753-11758. doi:10.1073/ pnas.1733027100 Dodd, K. M. and Tee, A. R. (2012). Leucine and mTORC1: a complex relationship. Am. J. Physiol. Endocrinol. Metab. 302, E1329-E1342. doi:10.1152/ajpendo. 00525.2011 Dou, X., Liu, Y., Cao, Y., Zhang, Y., Fu, X., Deng, J. and Tan, B. (2023). Effects of dietary lysine level on growth performance and protein metabolism in juvenile leopard coral grouper (Plectropomus leopardus). Aquaculture Nutr. 2023, 1017222. doi:10.1155/2023/1017222 Dragano, N. R. V., Solon, C., Ramalho, A. F., De Moura, R. F., Razolli, D. S., Christiansen, E., Azevedo, C., Ulven, T. and Velloso, L. A. (2017). Polyunsaturated fatty acid receptors, GPR40 and GPR120, are expressed in the hypothalamus and control energy homeostasis and inflammation. J. Neuroinflammation 14, 91. doi:10.1186/s12974-017-0869-7 Feng, H., Peng, D., Liang, X.-F., Li, J., Luo, H., Tang, S. and Chai, F. (2022). Intracerebroventricular injection with octanoic acid activates hypothalamic fatty acid sensing systems and regulates appetite in Chinese perch Siniperca chuatsi. Fish. Sci. 88, 83-90. doi:10.1007/s12562-021-01570-1 Festuccia, W. T., Blanchard, P. -G., Belchior, T., Chimin, P., Paschoal, V. A., Magdalon, J., Hirabara, S. M., Simoes, D., St-Pierre, F., Carpinelli, A. et al. (2014). PPARγ activation attenuates glucose intolerance induced by mTOR inhibition with rapamycin in rats. Am. J. Physiol. Endocrinol. Metab. 306, E1046-E1054. doi:10.1152/ajpendo.00683.2013 Figueiredo-Silva, A. C., Kaushik, S., Terrier, F., Schrama, J. W., Mé dale, F. and Geurden, I. (2012a). Link between lipid metabolism and voluntary food intake in rainbow trout fed coconut oil rich in medium-chain TAG. Brit. J. Nutr. 107, 1714-1725. doi:10.1017/S0007114511004739 Figueiredo-Silva, A. C., Saravanan, S., Schrama, J. W., Kaushik, S. and Geurden, I. (2012b). Macronutrient-induced differences in food intake relate with hepatic oxidative metabolism and hypothalamic regulatory neuropeptides in rainbow trout (Oncorhynchus mykiss). Physiol. Behav. 106, 499-505. doi:10. 1016/j.physbeh.2012.03.027 Figueiredo-Silva, A. C., Saravanan, S., Schrama, J. W., Panserat, S., Kaushik, S. and Geurden, I. (2013). A comparative study of the metabolic response in rainbow trout and Nile tilapia to changes in dietary macronutrient composition. Br. J. Nutr. 109, 816-826. doi:10.1017/s000711451200205x Fioramonti, X., Contié , S., Song, Z., Routh, V. H., Lorsignol, A. and Pé nicaud, L. (2007). Characterization of glucosensing neuron subpopulations in the arcuate nucleus. Integration in neuropeptide Y and pro-opio melanocortin networks? Diabetes 56, 1219-1227. doi:10.2337/db06-0567 Forsman, A. and Ruohonen, K. (2009). Dynamics of protein and lipid intake regulation of rainbow trout studied with a wide lipid range of encapsulated diets and self-feeders. Physiol. Behav. 96, 85-90. doi:10.1016/j.physbeh.2008.08.018 13 Journal of Experimental Biology REVIEW Fromentin, G., Darcel, N., Chaumontet, C., Marsset-Baglieri, A., Nadkarni, N. and Tom, D. (2012). Peripheral and central mechanisms involved in the control of food intake by dietary amino acids and proteins. Nutr. Res. Rev. 25, 29-39. doi:10. 1017/S0954422411000175 Gao, S., Moran, T. H., Lopaschuk, G. D. and Butler, A. A. (2013). Hypothalamic malonyl-CoA and the control of food intake. Physiol. Behav. 122, 17-24. doi:10. 1016/j.physbeh.2013.07.014 Gé lineau, A., Corraze, G., Boujard, T., Larroquet, L. and Kaushik, S. (2001). Relation between dietary lipid level and voluntary feed intake, growth, nutrient gain, lipid deposition and hepatic lipogenesis in rainbow trout. Reprod. Nutr. Dev. 41, 487-503. doi:10.1051/rnd:2001103 Gong, G., Xue, M., Wang, J., Wu, X.-F., Zheng, Y.-H., Han, F., Liang, X.-F. and Su, X.-O. (2014). The regulation of gluconeogenesis in the Siberian sturgeon (Acipenser baerii) affected later in life by a short-term high-glucose programming during early life. Aquaculture 436, 127-136. doi:10.1016/j.aquaculture.2014.10. 044 Gong, N., Jö nsson, E. and Bjö rnsson, B. T. (2016). Acute anorexigenic action of leptin in rainbow trout is mediated by the hypothalamic Pi3k pathway. J. Mol. Endocrinol. 56, 227-238. doi:10.1530/JME-15-0279 Gonzá lez, J. A., Reimann, F. and Burdakov, D. (2009). Dissociation between sensing and metabolism of glucose in sugar sensing neurones. J. Physiol. 587, 41-48. doi:10.1113/jphysiol.2008.163410 Gross, D. N., Wan, M. and Birnbaum, M. J. (2009). The role of FOXO in the regulation of metabolism. Curr. Diabetes Rep. 9, 208-214. doi:10.1007/s11892009-0034-5 Han, J., Liang, X., Guo, Y., Wu, X., Li, Z. and Hong, T. (2022). Agouti-related protein as the glucose signaling sensor in the central melanocortin circuits in regulating fish food intake. Front. Endocrinol. 13, 1010472. doi:10.3389/fendo. 2022.1010472 Hasebe, M., Kanda, S. and Oka, Y. (2016). Female-specific glucose sensitivity of GnRH1 neurons leads to sexually dimorphic inhibition of reproduction in medaka. Endocrinology 157, 4318-4329. doi:10.1210/en.2016-1352 Heeley, N. and Blouet, C. (2016). Central amino acid sensing in the control of feeding behavior. Front. Endocrinol. 7, 148. doi:10.3389/fendo.2016.00148 Heeley, N., Kirwan, P., Darwish, T., Arnaud, M., Evans, M. L., Merkle, F. T., Reimann, F., Gribble, F. M. and Blouet, C. (2018). Rapid sensing of L-leucine by human and murine hypothalamic neurons: Neurochemical and mechanistic insights. Mol. Metab. 10, 14-27. doi:10.1016/j.molmet.2018.01.021 Herrera Moro Chao, D., Argmann, C., Van Eijk, M., Boot, R. G., Ottenhoff, R., Van Roomen, C., Foppen, E., Siljee, J. E., Unmehopa, U. A., Kalsbeek, A. et al. (2016). Impact of obesity on taste receptor expression in extra-oral tissues: emphasis on hypothalamus and brainstem. Sci. Rep. 6, 29094. doi:10.1038/ srep29094 Hevrøy, E. M., Waagbø, R., Torstensen, B. E., Takle, H., Stubhaug, I., Jørgensen, S. M., Torgersen, T., Tvenning, L., Susort, S., Breck, O. et al. (2012). Ghrelin is involved in voluntary anorexia in Atlantic salmon raised at elevated sea temperatures. Gen. Comp. Endocrinol. 175, 118-134. doi:10.1016/ j.ygcen.2011.10.007 Higuchi, S., Irie, K., Yamaguchi, R., Katsuki, M., Araki, M., Ohji, M., Hayakawa, K., Mishima, S., Akitake, Y., Matsuyama, K. et al. (2012). Hypothalamic 2arachidonoylglycerol regulates multistage process of high-fat diet preferences. PLoS ONE 7, e38609. doi:10.1371/journal.pone.0038609 Hu, Z., Cha, S. H., Chohnan, S. and Lane, M. D. (2011). Hypothalamic malonylCoA as a mediator of feeding behavior. Proc. Natl. Acad. Sci. USA 100, 12624-12629. doi:10.1073/pnas.1834402100 Hu, F., Xu, Y. and Liu, F. (2016). Hypothalamic roles of mTOR complex I: integration of nutrient and hormone signals to regulate energy homeostasis. Am. J. Physiol. Endocrinol. Metab. 310, E994-E1002. doi:10.1152/ajpendo.00121.2016 Hundal, H. S. and Taylor, P. M. (2009). Amino acid transceptors: Gate keepers of nutrient exchange and regulators of nutrient signaling. Am. J. Physiol. Endocrinol. Metab. 296, E603-E613. doi:10.1152/ajpendo.91002.2008 Ichimura, A., Hirasawa, A., Hara, T. and Tsujimoto, G. (2009). Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat. 89, 82-88. doi:10.1016/j.prostaglandins.2009.05.003 Jewell, J. L. and Guan, K. (2013). Nutrient signaling to mTOR and cell growth. Trends Biochem. Sci. 38, 233-242. doi:10.1016/j.tibs.2013.01.004 Jin, J., Mé dale, F., Kamalam, B. S., Aguirre, P., Vé ron, V. and Panserat, S. (2014). Comparison of glucose and lipid metabolic gene expressions between fat and lean lines of rainbow trout after a glucose load. PLoS ONE 9, e105548. doi:10. 1371/journal.pone.0105548 Johansen, S. J. S., Ekli, M. and Jobling, M. (2002). Is there lipostatic regulation of feed intake in Atlantic salmon Salmo salar L.?: Lipostatic regulation of feeding in salmon. . Aquaculture Res. 33, 515-524. doi:10.1046/j.1365-2109.2002.00736.x Johansen, S. J. S., Sveier, H. and Jobling, M. (2003). Lipostatic regulation of feed intake in Atlantic salmon Salmo salar L. defending adiposity at the expense of growth? Aquaculture Res. 34, 1245-1245. doi:10.1046/j.1365-2109.2003. 00821.x Jö rgens, K., Stoll, S. J., Pohl, J., Fleming, T. H., Sticht, C., Nawroth, P. P., Hammes, H.-P. and Kroll, J. (2015). High tissue glucose alters intersomitic blood Journal of Experimental Biology (2024) 227, jeb247410. doi:10.1242/jeb.247410 vessels in zebrafish via methylglioxal targeting the VEGF receptor signalling cascade. Diabetes 64, 213-225. doi:10.2337/db14-0352 Jurczyk, A., Roy, N., Bajwa, R., Gut, P., Lipson, K., Yang, C., Covassin, L., Racki, W. J., Rossini, A. A., Phillips, N. et al. (2011). Dynamic glucoregulation and mammalian-like responses to metabolic and developmental disruption in zebrafish. Gen. Comp. Endocrinol. 170, 334-345. doi:10.1016/j.ygcen.2010. 10.010 Kamalam, B. S., Medale, F., Kaushik, S., Polakof, S., Skiba-Cassy, S. and Panserat, S. (2012). Regulation of metabolism by dietary carbohydrate in two lines of rainbow trout divergently selected for muscle fat content. J. Exp. Biol. 215, 2567-2578. doi:10.1242/jeb.070581 Kaushik, S., Mé dale, F., Fauconneau, B. and Blanc, D. (1989). Effect of digestible carbohydrates on protein - Energy utilization and on glucose metabolism in rainbow trout (Salmo gairdneri R). Aquaculture 79, 63-74. doi:10.1016/00448486(89)90446-8 Kim, H. -I. and Ahn, Y. -H. (2004). Role of peroxisome proliferator-activated receptor-γ in the glucose-sensing apparatus of liver and β-cells. Diabetes 53, S60-S65. doi:10.2337/diabetes.53.2007.S60 Kim, E.-K., Kleman, A. K. and Ronnett, G. V. (2007). Fatty acid synthase gene regulation in primary hypothalamic neurons. Neurosci. Lett. 423, 200-204. doi:10. 1016/j.neulet.2007.06.056 Kinnamon, S. C. (2012). Taste receptor signalling - from tongues to lungs. Acta Physiol. 204, 158-168. doi:10.1111/j.1748-1716.2011.02308.x Kong, D., Vong, L., Parton, L. E., Ye, C., Tong, Q., Hu, X., Choi, B., Brü ning, J. C. and Lowell, B. B. (2010). Glucose stimulation of hypothalamic MCH neurons involves KATP channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metab. 12, 545-552. doi:10.1016/j.cmet.2010.09.013 Krogdahl, A., Sundby, A. and Olli, J. J. (2004). Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss) digest and metabolize nutrients differently. Effects of water salinity and dietary starch level. Aquaculture 229, 335-360. doi:10. 1016/S0044-8486(03)00396-X Lage, R., Vá zquez, M. J., Varela, L., Saha, A. K., Vidal-Puig, A., Nogueiras, R., Dié guez, C. and Ló pez, M. (2010). Ghrelin effects on neuropeptides in the rat hypothalamus depend on fatty acid metabolism actions on BSX but not on gender. FASEB J. 24, 2670-2679. doi:10.1096/fj.09-150672 Lai, F., Comesaña, S., Gomes, A. S., Flatejord, D., Tolås, I., Espe, M., De Santis, C., Hartviksen, M. B., Verri, T., Soengas, J. L. et al. (2023). Anorectic role of high dietary leucine in farmed Atlantic salmon (Salmo salar L.): Effects on feed intake, growth, amino acid transporters and appetite-control neuropeptides. Aquaculture 566, 739204. Le Foll, C., Irani, B. G., Magnan, C., Dunn-Meynell, A. A. and Levin, B. E. (2009). Characteristics and mechanisms of hypothalamic neuronal fatty acid sensing. Am. J. Physiol. Regul. Integ. Comp. Physiol. 297, R655-R664. doi:10.1152/ ajpregu.00223.2009 Lee, B., Lee, S., Lee, S.-K. and Lee, J. W. (2016). The LIM-homeobox transcription factor Isl1 plays crucial roles in the development of multiple arcuate nucleus neurons. Development 143, 3763-3773. doi:10.1242/dev.133967 Levin, B. E., Routh, V. H., Kang, L., Sanders, N. M. and Dunn-Meynell, A. A. (2004). Neuronal glucosensing. What do we know after 50 years? Diabetes 53, 2521-2528. doi:10.2337/diabetes.53.10.2521 Liang, X., Han, J., Xue, M., Yu, H., Huang, H., Wu, X., Zheng, Y., Qin, Y. and Liang, X. (2019). Growth and feed intake regulation responses to anorexia, adaptation and fasting in Japanese seabass, Lateolabrax japonicas when fishmeal is totally replaced by plant protein. Aquaculture 498, 528-538. doi:10. 1016/j.aquaculture.2018.09.010 Librá n-Pé rez, M., Polakof, S., Ló pez-Patino, ̃ M. A., Mı́guez, J. M. and Soengas, J. L. (2012). Evidence of a metabolic fatty acid-sensing system in the hypothalamus and Brockmann bodies of rainbow trout: implications in food intake regulation. Am. J. Physiol. Regul. Integ. Comp. Physiol. 302, R1340-R1350. doi:10.1152/ajpregu.00070.2012 Librá n-Pé rez, M., Ló pez-Patino, ̃ M. A., Mı́guez, J. M. and Soengas, J. L. (2013b). Oleic acid and octanoic acid sensing capacity in rainbow trout Oncorhynchus mykiss is direct in hypothalamus and Brockmann bodies. PLoS ONE 8, e59507. doi:10.1371/journal.pone.0059507 Librá n-Pé rez, M., Otero-Rodiño, C., Ló pez-Patiño, M. A., Mı́guez, J. M. and Soengas, J. L. (2014a). Central administration of oleate or octanoate activates hypothalamic fatty acid sensing and inhibits food intake in rainbow trout. Physiol. Behav. 129, 272-279. doi:10.1016/j.physbeh.2014.02.061 Librá n-Pé rez, M., Velasco, C., Ló pez-Patino, ̃ M. A., Mı́guez, J. M. and Soengas, J. L. (2014b). Counter-regulatory response to a fall in circulating fatty acid levels in rainbow trout. Possible involvement of the hypothalamus-pituitary-interrenal axis. PLoS ONE 9, e113291. doi:10.1371/journal.pone.0113291 Librá n-Pé rez, M., Geurden, I., Dias, K., Corraze, G., Panserat, S. and Soengas, J. L. (2015a). Feeding rainbow trout with a lipid-enriched diet: effects on fatty acid sensing, regulation of food intake and cellular signalling pathways. J. Exp. Biol. 218, 2610-2619. doi:10.1242/jeb.123802 Librá n-Pé rez, M., Otero-Rodino, ̃ C., Ló pez-Patino, ̃ M. A., Mı́guez, J. M. and Soengas, J. L. (2015b). Effects of intracerebroventricular treatment with oleate or octanoate on fatty acid metabolism in Brockmann bodies and liver of rainbow trout. Aquaculture Nutr. 21, 194-205. doi:10.1111/anu.12158 14 Journal of Experimental Biology REVIEW Librá n-Pé rez, M., Velasco, C., Otero-Rodino, ̃ C., Ló pez-Patino, ̃ M. A., Mı́guez, J. M. and Soengas, J. L. (2015c). Metabolic response in liver and Brockmann bodies of rainbow trout to inhibition of lipolysis; possible involvement of the hypothalamus–pituitary–interrenal (HPI) axis. J. Comp. Physiol. B 185, 413-423. doi:10.1007/s00360-015-0894-8 Liu, H., Xu, Y., Wang, Y., Zhong, S., Wang, M., Lin, P., Li, H. and Liu, Z. (2017). Cd36 is a candidate lipid sensor involved in the sensory detection of fatty acid in zebrafish. Physiol. Behav. 182, 34-39. doi:10.1016/j.physbeh.2017.09.015 Liu, D., Deng, K., Sampath, W. W. H. A., Gu, Z., Pan, M., Zhang, Y., Zhang, W. B. and Mai, K. (2019). Responses of glucosensing system to glucose in Japanese flounder Paralichthys olivaceus fed diets with different carbohydrate content. Comp. Biochem. Physiol. B 232, 72-78. doi:10.1016/j.cbpb.2019.03.001 Lockie, S. H., Stark, R., Mequinion, M., Ching, S., Kong, D., Spanswick, D. C., Lawrence, A. J. and Andrews, Z. B. (2019). Glucose availability predicts the feeding response to ghrelin in male mice, an effect dependent on AMPK in AgRP neurons. Endocrinology 159, 3605-3614. doi:10.1210/en.2018-00536 Ló pez, M., Tovar, S., Vá zquez, M. J., Nogueiras, R., Señarı́s, R. and Dié guez, C. (2005). Sensing the fat: Fatty acid metabolism in the hypothalamus and the melanocortin system. Peptides 26, 1753-1758. doi:10.1016/j.peptides.2004. 11.025 Ló pez, M., Lelliott, C. J. and Vidal-Puig, A. (2007). Hypothalamic fatty acid metabolism: a housekeeping pathway that regulates food intake. BioEssays 29, 248-261. doi:10.1002/bies.20539 Ló pez-Gambero, A. J., Martı́nez, F., Salazar, K., Cifuentes, M. and Nualart, F. (2019). Brain glucose-sensing mechanism and energy homeostasis. Mol. Neurobiol. 56, 769-796. doi:10.1007/s12035-018-1099-4 Lueders, B., Kanney, B. C., Krone, M. J., Gannon, N. P. and Vaughan, R. A. (2022). Effect of branched-chain amino acids on food intake and indicators of hunger and satiety- a narrative summary. Human Nutr. Metab. 30, 200168. doi:10. 1016/j.hnm.2022.200168 Luo, H., Liang, X.-F., Li, J., Zhang, Y., Xiao, Q., Peng, B. and Zhang, Z. (2020). Effect of long-chain saturated and unsaturated fatty acids on hypothalamic fatty acid sensing in Chinese perch (Siniperca chuatsi). Comp. Biochem. Physiol. B 241, 110395. doi:10.1016/j.cbpb.2019.110395 Luo, L., Xue, M., Vachot, C., Geurden, I. and Kaushik, S. (2014). Dietary medium chain fatty acids from coconut oil have little effects on postprandial plasma metabolite profiles in rainbow trout (Oncorhynchus mykiss). Aquaculture 420-421, 24-31. doi:10.1016/j.aquaculture.2013.10.024 Marty, N., Dallaporta, M. and Thorens, B. (2007). Brain glucose sensing, counteregulation, and energy homeostasis. Physiology 22, 241-251. doi:10. 1152/physiol.00010.2007 Miao, Y., Liu, Y., Cui, K., Ding, T., Xu, N., Chen, Q., Fang, W., Mai, K. and Ai, Q. (2021). Effects of dietary arginine on growth, activity of digestive enzymes, GCN2–ATF4 signalling pathway and nutritional metabolism-related gene expression of large yellow croaker (Larimichthys crocea) larvae. Aquaculture Nutr. 27, 2333-2343. doi:10.1111/anu.13366 Migrenne, S., Le Foll, C., Levin, B. E. and Magnan, C. (2011). Brain lipid sensing and nervous control of energy balance. Diabetes Metab. 37, 83-88. doi:10.1016/j. diabet.2010.11.001 Mobbs, C. V., Isoda, F., Makimura, H., Mastaitis, J., Mizuno, T., Shu, I.-W., Yen, K. and Yang, X.-J. (2005). Impaired glucose signaling as a cause of obesity and the metabolic syndrome: the glucoadipostatic hypothesis. Physiol. Behav. 85, 2-23. doi:10.1016/j.physbeh.2005.04.005 Morais, S. (2017). The physiology of taste in fish: potential implications for feeding stimulation and gut chemical sensing. Rev. Fish. Sci. Aquaculture 25, 133-149. doi:10.1080/23308249.2016.1249279 Morrison, C. D. and Laeger, T. (2015). Protein-dependent regulation of feeding and metabolism. Trends Endocrinol. Metab. 26, 256-262. doi:10.1016/j.tem.2015. 02.008 Morgan, K., Obici, S. and Rossetti, L. (2004). Hypothalamic responses to longchain fatty acids are nutritionally regulated. J. Biol. Chem. 279, 31139-31148. doi:10.1074/jbc.M400458200 Morton, G. J., Meek, T. H. and Schwartz, M. W. (2014). Neurobiology of food intake in health and disease. Nature Rev. Neurosci. 15, 367-378. doi:10.1038/nrn3745 Mountjoy, P. D., Bailey, S. J. and Rutter, G. A. (2007). Inhibition by glucose or leptin of hypothalamic neurons expressing neuropeptide Y requires changes in AMP-activated protein kinase activity. Diabetologia 50, 168-177. doi:10.1007/ s00125-006-0473-3 Mourente, G. and Tocher, D. R. (1992). Lipid class and fatty acid composition of brain lipids from Atlantic herring (Clupea harengus) at different stages of development. Mar. Biol. 112, 553-558. doi:10.1007/BF00346172 Narnaware, Y. and Peter, R. E. (2002). Influence of diet composition on food intake and neuropeptide Y (NPY) gene expression in goldfish brain. Regul. Pept. 103, 75-83. doi:10.1016/s0167-0115(01)00342-1 Navarro, I. and Gutié rrez, J. (1995). Chapter 17 Fasting and starvation. Biochem. Mol. Biol. Fish. 4, 393-434. Nipu, N., Antomagesh, F., Faught, E. and Vijayan, M. M. (2022). Glucocorticoid receptor activation reduces food intake independent of hyperglycemia in zebrafish. Scientif. Rep. 12, 15677. doi:10.1038/s41598-022-19572-z Journal of Experimental Biology (2024) 227, jeb247410. doi:10.1242/jeb.247410 Niu, X., Qian, X., Feng, H., Kui, Y., Dong, L., Weijun, C. and Jidan, Y. (2021). Growth and metabolic responses of grouper juveniles (Epinephelus coioides) fed diets containing varying levels of leucine. Aquaculture 534, 736281. doi:10.1016/ j.aquaculture.2020.736281 Nogueiras, R., Ló pez, M., Lage, R., Perez-Tilve, D., Pfluger, P., Mendieta-Zeró n, H., Sakkou, M., Wiedmer, P., Benoit, S. C., Datta, R. et al. (2008). Bsx, a novel hypothalamic factor linking feeding with locomotor activity, is regulated by energy availability. Endocrinology 149, 3009-3015. doi:10.1210/en.2007-1684 Obici, S., Feng, Z., Morgan, K., Stein, D., Karkanias, G. and Rossetti, L. (2002). Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51, 271-275. doi:10.2337/diabetes.51.2.271 Ogunnowo-Bada, E., Heeley, N., Brochard, L. and Evans, M. L. (2014). Brain glucose sensing, glucokinase and neural control of metabolism and islet function. Diabetes Obes. Metab. 16, 26-32. doi:10.1111/dom.12334 Oh, Y. T., Oh, K.-S., Kang, I. and Youn, J. H. (2012). A fall in plasma free fatty acid (FFA) level activates the hypothalamic-pituitary-adrenal axis independent of plasma glucose: Evidence for brain sensing of circulating FFA. Endocrinology 153, 3587-3592. doi:10.1210/en.2012-1330 Oh, Y. T., Kim, J., Kang, I. and Youn, J. H. (2014). Regulation of hypothalamicpituitary-adrenal axis by circulating free fatty acids in male Wistar rats: role of individual free fatty acids. Endocrinology 155, 923-931. doi:10.1210/ en.2013-1700 Oh, Y. T., Oh, H. H., Nguyen, A.-K., Choi, C. S. and Youn, J. H. (2016). Circulating free fatty acids inhibit food intake in an oleate-specific manner in rats. Physiol. Behav. 167, 194-201. doi:10.1016/j.physbeh.2016.09.015 O’Malley, D., Reimann, F., Simpson, A. K. and Gribble, F. M. (2006). Sodiumcoupled glucose cotransporters contribute to hypothalamic glucose sensing. Diabetes 55, 3381-3386. doi:10.2337/db06-0531 Otero-Rodiño, C., Librá n-Pé rez, M., Velasco, C., Ló pez-Patiño, M. A., Mı́guez, J. M. and Soengas, J. L. (2015a). Evidence for the presence of glucosensor mechanisms not dependent on glucokinase in hypothalamus and hindbrain of rainbow trout (Oncorhynchus mykiss). PLoS ONE 10, e0128603. doi:10.1371/ journal.pone.0128603 Otero-Rodino, ̃ C., Librá n-Pé rez, M., Velasco, C., Álvarez-Otero, R., Ló pezPatiño, M. A., Mı́guez, J. M. and Soengas, J. L. (2015b). Response of lactate metabolism in brain glucosensing areas of rainbow trout (Oncorhynchus mykiss) to changes in glucose levels. J. Comp. Physiol. B 185, 869-882. doi:10.1007/ s00360-015-0932-6 Otero-Rodino, ̃ C., Velasco, C., Álvarez-Otero, R., Ló pez-Patino, ̃ M. A., Mı́guez, J. M. and Soengas, J. L. (2016). In vitro evidence supports the presence of glucokinase-independent glucosensing mechanisms in hypothalamus and hindbrain of rainbow trout. J. Exp. Biol. 219, 1750-1759. doi:10.1242/jeb.137737 Otero-Rodiño, C., Velasco, C., Álvarez-Otero, R., Ló pez Patiño, M. A., Mı́guez, J. M. and Soengas, J. L. (2017). Changes in the levels and phosphorylation status of Akt, AMPK, CREB and FoxO1 in hypothalamus of rainbow trout under conditions of enhanced glucosensing activity. J. Exp. Biol. 220, 4410-4417. doi:10.1242/jeb.165159 Otero-Rodiño, C., Rocha, A., Sá nchez, E., Álvarez-Otero, R., Soengas, J. L. and Cerdá -Reverter, J. M. (2019a). Sensing glucose in the central melanocortin circuits of rainbow trout: a morphological study. Front. Endocrinol. 10, 254. doi:10. 3389/fendo.2019.00254 Otero-Rodino, ̃ C., Conde-Sieira, M., Comesana, ̃ S., Álvarez-Otero, R., Ló pez+ + Patino, ̃ M. A., Mı́guez, J. M. and Soengas, J. L. (2019b). Na /K -ATPase is involved in the regulation of food intake in rainbow trout but apparently not through brain glucosensing mechanisms. Physiol. Behav. 209, 112617. doi:10.1016/ j.physbeh.2019.112617 Park, S., Kim, D. S. and Daily, J. W. (2011). Central infusion of ketone bodies modulates body weight and hepatic insulin sensititivy by modifying hypothalamic leptin and insulin signalling pathways in type 2 diabetic rats. Brain Res. 1401, 95-103. doi:10.1016/j.brainres.2011.05.040 Pellerin, L. and Magistretti, P. J. (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 91, 10625-10629. Penney, C. C. and Volkoff, H. (2014). Peripheral injections of cholecystokinin, apelin, ghrelin and orexin in cavefish (Astyanax fasciatus mexicanus): effects on feeding and on the brain expression levels of tyrosine hydroxylase, mechanistic target of rapamycin and appetite-related hormones. Gen. Comp. Endocrinol. 196, 34-40. doi:10.1016/j.ygcen.2013.11.015 Peragó n, J., Barroso, J. B., Garcı́a-Salguero, L., de la Higuera, M. and Lupiá ñez, J. A. (2000). Dietary alterations in protein, carbohydrates and fat increase liver protein-turnover rate and decrease overall growth rate in the rainbow trout (Oncorhynchus mykiss). Mol. Cell. Biochem. 209, 97-104. doi:10.1023/ a:1007130906365 Picard, A., Moullé , V. S., Le Foll, C., Cansell, C., Vé ret, J., Coant, N., Le Stunff, H., Migrenne, S., Luquet, S., Cruciani-Guglielmacci, C. et al. (2014). Physiological and pathophysiological implications of lipid sensing in the brain. Diabetes Obes. Metab 16 Suppl. 1, 49-55. doi:10.1111/dom.12335 Pisera-Fuster, A., Zwiller, J. and Bernabeu, R. (2021). Methionine supplementation abolishes nicotine-induced place preference in zebrafish: a 15 Journal of Experimental Biology REVIEW behavioral and molecular analysis. Mol. Neurobiol. 58, 2590-2607. doi:10.1007/ s12035-020-02260-2 Polakof, S. and Soengas, J. L. (2008). Involvement of lactate in glucose metabolism and glucosensing function in selected tissues of rainbow trout. J. Exp. Biol. 211, 1075-1086. doi:10.1242/jeb.014050 Polakof, S., Mı́guez, J. M., Moon, T. W. and Soengas, J. L. (2007a). Evidence for the presence of a glucosensor in hypothalamus, hindbrain, and Brockmann bodies of rainbow trout. Am. J. Physiol. Reg. Integr. Comp. Physiol. 292, 1657-1666. doi:10.1152/ajpregu.00525.2006 Polakof, S., Mı́guez, J. M. and Soengas, J. L. (2007b). In vitro evidences for glucosensing capacity and mechanisms in hypothalamus, hindbrain, and Brockmann bodies of rainbow trout. Am. J. Physiol. Reg. Integr. Comp. Physiol. 293, 1410-1420. doi:10.1152/ajpregu.00283.2007 Polakof, S., Mı́guez, J. M. and Soengas, J. L. (2008a). Changes in food intake and glucosensing function of hypothalamus and hindbrain in rainbow trout subjected to hyperglycemic or hypoglycemic conditions. J. Comp. Physiol. A 194, 829-839. doi:10.1007/s00359-008-0354-y Polakof, S., Mı́guez, J. M. and Soengas, J. L. (2008b). Dietary carbohydrates induce changes in glucosensing capacity and food intake in rainbow trout. Am. J. Physiol. Reg. Integr. Comp. Physiol. 295, 478-489. doi:10.1152/ajpregu. 00176.2008 Polakof, S., Panserat, S., Plagnes-Juan, E. and Soengas, J. L. (2008c). Altered dietary carbohydrates significantly affect gene expression of the major glucosensing components in Brockmannn bodies and hypothalamus of rainbow trout. Am. J. Physiol. Reg. Integr. Comp. Physiol. 295, 1077-1088. doi:10.1152/ ajpregu.90476.2008 Polakof, S., Rodrı́guez-Alonso, M. and Soengas, J. L. (2009). Immunohistochemical localization of glucokinase in rainbow trout brain. Comp. Biochem. Physiol. A 153, 352-358. doi:10.1016/j.cbpa.2009.03.015 Polakof, S., Panserat, S., Craig, P. M., Martyres, D. J., Plagnes-Juan, E., Savari, S., Aris-Brosou, S. and Moon, T. W. (2011). The metabolic consequences of hepatic AMP-kinase phosphorylation in rainbow trout. PLoS ONE 6, e20228. doi:10.1371/journal.pone.0020228 Purpera, M. N., Shen, L., Taghavi, M., Mü nzberg, H., Martin, R. J., Hutson, S. M. and Morrison, C. D. (2012). Impaired branched chain amino acid metabolism alters feeding behavior and increases orexigenic neuropeptide expression in the hypothalamus. J. Endocrinol. 212, 85-94. doi:10.1530/JOE-11-0270 Rasmussen, R. S., Ostenfeld, T. H., Rønsholdt, B. and McLean, E. (2000). Manipulation of end-product quality of rainbow trout with finishing diets: Finishing diets and trout quality. Aquaculture Nutr. 6, 17-23. doi:10.1046/j.1365-2095.2000. 00119.x Ren, X., Zhou, L., Terwiliger, R., Newton, S. S. and de Araujo, I. E. (2009). Sweet taste signaling functions as a hypothalamic glucose sensor. Front. Integr. Neurosci. 3, 1-15. doi:10.3389/neuro.07.012.2009 Roh, E., Song, D. K. and Kim, M.-S. (2016). Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp. Mol. Med. 48, e216. Roy, J., Vigor, C., Vercauteren, J., Reversat, G., Zhou, B., Surget, A., Larroquet, L., Lanuque, A., Sandres, F., Terrier, F. et al. (2020). Characterization and modulation of brain lipids content of rainbow trout fed with 100% plant based diet rich in omega-3 long chain polyunsaturated fatty acids DHA and EPA. Biochimie 178, 137-147. doi:10.1016/j.biochi.2020.06.010 Ruibal, C., Soengas, J. L. and Aldegunde, M. (2002). Brain serotonin and the control of food intake in rainbow trout (Oncorhynchus mykiss): effects of changes in plasma glucose levels. J. Comp. Physiol. A 188, 479-484. doi:10.1007/s00359002-0320-z Sala-Rabanal, M., Gallardo, M. A., Sá nchez, J. and Planas, J. M. (2004). Nadependent D-glucose transport by intestinal brush border membrane vesicles from gilthead sea bream (Sparus aurata). J. Membrane Biol. 201, 85-96. doi:10. 1007/s00232-004-0710-y Sá nchez-Muros, M. J., Garcı́a-Rejó n, L., Garcı́a-Salguero, L., de la Higuera, M. and Lupiá nez, J. A. (1998). Long-term nutritional effects on the pimary liver and ̃ kidney metabolism in rainbow trout. Adaptative response to starvation and highprotein, carbohydrate-free diet to glutamate dehydrogenase and alanine aminotransferase kinetics. Int. J. Biochem. 30, 55-63. doi:10.1016/s13572725(97)00100-3 Saravanan, S., Geurden, I., Figueiredo-Silva, A. C., Kaushik, S., Verreth, J. and Schrama, J. W. (2013). Voluntary feed intake in rainbow trout is regulated by dietinduced differences in oxygen use. J. Nutr. 143, 781-787. doi:10.3945/jn.112. 173062 Sargent, J. R., Tocher, D. R. and Bell, J. G. (2002). The lipids. In Fish Nutrition (ed. J. E. Halverand, R. W. Hardy), pp. 182-258. San Diego, USA: Academic Press. Schö nfeld, P. and Wojtczak, L. (2008). Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radicals Biol. Med. 45, 231-241. doi:10.1016/j.freeradbiomed.2008.04.029 Schwartz, M. W., Woods, S. C., Porte, D., Seeley, R. J. and Baskin, D. G. (2000). Central nervous system control of food intake. Nature 404, 661-671. doi:10.1038/ 35007534 Seth, H. and Axelsson, M. (2010). Sympathetic, parasympathetic and enteric regulation of the gastrointestinal vasculature in rainbow trout (Oncorhynchus Journal of Experimental Biology (2024) 227, jeb247410. doi:10.1242/jeb.247410 mykiss) under normal and postprandial conditions. J. Exp. Biol. 213, 3118-3126. doi:10.1242/jeb.043612 Shearer, K. D., Silverstein, J. T. and Plisetskaya, E. M. (1997). Role of adiposity in food intake control of juvenile chinook salmon (Oncorhynchus tshawytscha). Comp. Biochem. Physiol. A 118, 1209-1215. doi:10.1016/S03009629(97)86801-6 Silverstein, J. T., Shearer, K. D., Dickhoff, W. W. and Plisetskaya, E. M. (1999). Regulation of nutrient intake and energy balance in salmon. Aquaculture 177, 161-169. doi:10.1016/S0044-8486(99)00076-9 Skiba-Cassy, S., Panserat, S., Larquier, M., Dias, K., Surget, A., Plagnes-Juan, E., Kaushik, S. and Seiliez, I. (2013). Apparent low ability of liver and muscle to adapt to variation of dietary carbohydrate:protein ratio in rainbow trout (Oncorhynchus mykiss). Brit. J. Nutr. 109, 1359-1372. doi:10.1017/ S0007114512003352 Skiba-Cassy, S., Geurden, I., Panserat, S. and Seiliez, I. (2016). Dietary methionine imbalance alters the transcriptional regulation of genes involved in glucose, lipid and amino acid metabolismin the liver of rainbow trout (Oncorhynchus mykiss). Aquaculture 454, 56-65. doi:10.1016/j.aquaculture. 2015.12.015 Soengas, J. L. (2014). Contribution of glucose- and fatty acid sensing systems to the regulation of food intake in fish. A review. Gen. Comp. Endocrinol. 205, 36-48. doi:10.1016/j.ygcen.2014.01.015 Soengas, J. L. (2021). Integration of nutrient sensing in fish hypothalamus. Front. Neurosci. 15, 653928. Soengas, J. L. and Polakof, S. (2013). Glucosensing in rainbow trout. In Trout: From Physiology to Conservation (ed. S. Polakof and T. W. Moon), pp. 155-177 Nova Science Publishers. Soengas, J. L., Cerdá -Reverter, J. M. and Delgado, M. J. (2018). Central regulation of food intake in fish: an evolutionary perspective. J. Mol. Endocrinol. 60, R171-R199. doi:10.1530/JME-17-0320 Sohn, J. W. (2015). Network of hypothalamic neurons that control appetite. BMB Rep. 48, 229-233. doi:10.5483/BMBRep.2015.48.4.272 Su, Y., Lam, T. K. T., He, W., Pocai, A., Bryan, J., Aguilar-Bryan, L. and Gutié rrez-Juá rez, R. (2012). Hypothalamic leucine metabolism regulates liver glucose production. Diabetes 61, 85-93. doi:10.2337/db11-0857 Suá rez, M. D., Sanz, A., Bazoco, J. and Garcı́a-Gallego, M. (2002). Metabolic effects of changes in the dietary protein:carbohydrate ratio in eel (Anguilla anguilla) and trout (Oncorhynchus mykiss). Aquac. Int. 10, 143-156. doi:10.1023/ A:1021371104839 Subhedar, N., Varsagade, V. G., Singru, P. S., Thim, L. and Clausen, J. T. (2011). Cocaine- and amphetamine-regulated transcript peptide (CART) in the telencephalon of the catfish, Clarias gariepinus: distribution and response to fasting, 2-deoxy-D-glucose, glucose, insulin, and leptin treatments. J. Comp. Neurol. 519, 1281-1300. doi:10.1002/cne.22569 Sugiura, S. H., McDaniel, N. K. and Ferraris, R. P. (2003). In vivo fractional Pi absorption and NaPi-II mRNA expression in rainbow trout are upregulated by dietary P restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, 770-781. doi:10.1152/ajpregu.00127.2003 Tan, X., Lin, H., Huang, Z., Zhu, C., Wang, A., Qi, C. and Zhao, S. (2016). Effects of dietary leucine on growth performance, feed utilization, non-specific immune responses and gut morphology of juvenile golden pompano Trachinotus ovatus. Aquaculture 465, 100-107. doi:10.1016/j.aquaculture.2016.08.034 Tang, N., Li, Y., Li, Y., Liu, Y., Zhang, S., Xu, S., Wang, M., Wang, B., Chen, H., Zhang, X. et al. (2022). Molecular cloning, expression and appetite regulation function of adiponectin in Siberian sturgeon (Molecular cloning, expression and appetite regulation function of adiponectin in Siberian sturgeon (Acipenser baerii). International J. Biol. Macromol. 214, 360-369. doi:10.1016/j.ijbiomac.2022.06.097 Taylor, P. M. (2014). Role of amino acid transporters in amino acid sensing. American J. Clin. Nutr. 99, 223S-230S. doi:10.3945/ajcn.113.070086 Thorens, B. (2012). Sensing of glucose in the brain. In Appetite Control (ed. H. G. Joost), p. 277-293. Berlin: Springer-Verlag. Tian, J., Liu, W., Gao, W., Wu, F., Yu, L., Lu, X., Yang, C. G., Jiang, M. and Wen, H. (2017). Molecular cloning and gene/protein expression of FAT/CD36 from grass carp (Ctenopharyngodon idella) and the regulation of its expression by dietary energy. Fish Physiol. Biochem. 43, 875-888. doi:10.1007/s10695-017-0342-7 Timper, K. and Brü ning, J. C. (2017). Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis. Mod. Mech. 10, 679-689. doi:10. 1242/dmm.026609 Tocher, D. R. (2003). Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 11, 107-184. doi:10.1080/713610925 Usui, R., Yabe, D., Fauzi, M., Goto, H., Botagarova, A., Tokumoto, S., Tatsuoka, H., Tahara, Y., Kobayashi, S., Manabe, T. et al. (2019). GPR40 activation initiates store-operated Ca2+ entry and potentiates insulin secretion via the IP3R1/STIM1/Orai1 pathway in pancreatic β-cells. Scientif. Rep. 9, 15562. doi:10. 1038/s41598-019-52048-1 Varela, L., Vá zquez, M. J., Cordido, F., Nogueiras, R., Vidal-Puig, A., Dié guez, C. and Ló pez, M. (2011). Ghrelin and lipid metabolism: key partners in energy balance. J. Mol. Endocrinol. 46, R43-R63. doi:10.1677/JME-10-0068 Velasco, C., Librá n-Pé rez, M., Otero-Rodino, ̃ C., Ló pez-Patino, ̃ M. A., Mı́guez, J. M., Cerdá -Reverter, J. M. and Soengas, J. L. (2016a). Ghrelin modulates 16 Journal of Experimental Biology REVIEW hypothalamic fatty acid-sensing and control of food intake in rainbow trout. J. Endocrinol. 228, 25-37. doi:10.1530/JOE-15-0391 Velasco, C., Librá n-Pé rez, M., Otero-Rodiño, C., Ló pez-Patiño, M. A., Mı́guez, J. M. and Soengas, J. L. (2016b). Ceramides are involved in regulation of food intake in rainbow trout (Oncorhynchus mykiss). Am. J. Physiol. Regul. Integr. Comp. Physiol. 311, R658-R668. doi:10.1152/ajpregu.00201.2016 Velasco, C., Moreiras, G., Conde-Sieira, M., Leao, J. M., Mı́guez, J. M. and Soengas, J. L. (2017a). Ceramide counteracts the effects of ghrelin on the metabolic control of food intake in rainbow trout. J. Exp. Biol. 220, 2563-2576. doi:10.1242/jeb.159871 Velasco, C., Otero-Rodino, ̃ C., Comesana, ̃ S., Mı́guez, J. M. and Soengas, J. L. (2017b). Hypothalamic mechanisms linking fatty acid sensing and food intake regulation in rainbow trout. J. Mol. Endocrinol. 59, 377-390. doi:10.1530/JME-170148 Velasco, C., Bonacic, K., Soengas, J. L. and Morais, S. (2017c). Orally administered fatty acids enhance anorectic potential but do not activate central fatty acid sensing in Senegalese sole post-larvae. J. Exp. Biol. 220, 677-685. doi:10.1242/jeb.150979 Velasco, C., Blanco, A. M., Unniappan, S. and Soengas, J. L. (2018). The anorectic effect of central PYY1-36 treatment in rainbow trout (Oncorhynchus mykiss) is associated with changes in mRNAs encoding neuropeptides and parameters related to fatty acid sensing and metabolism. Gen. Comp. Endocrinol. 267, 137-145. doi:10.1016/j.ygcen.2018.06.015 Velasco, C., Comesana, ̃ S., Conde-Sieira, M., Mı́guez, J. M. and Soengas, J. L. (2019). Effects of CCK-8 and GLP-1 on fatty acid sensing and food intake regulation in trout. J. Mol. Endocrinol. 62, 101-116. doi:10.1530/JME-18-0212 Velasco, C., Conde-Sieira, M., Comesaña, S., Chivite, M., Dı́az-Rú a, A., Mı́guez, J. M. and Soengas, J. L. (2020). The long-chain fatty acid receptors FFA1 and FFA4 are involved in food intake regulation in fish brain. J. Exp. Biol. 223, jeb227330. doi:10.1242/jeb.227330 Velasco, C., Conde-Sieira, M., Comesaña, S., Chivite, M., Mı́guez, J. M. and Soengas, J. L. (2021). Role of the G protein-coupled receptors GPR84 and GPR119 in the central regulation of feed intake in rainbow trout. J. Exp. Biol. 224, jeb242360. doi:10.1242/jeb.242360 Vinnicombe, K. R. T. and Volkoff, H. (2022). Possible role of transcription factors (BSX, NKX2.1, IRX3 and SIRT1) in the regulation of appetite in goldfish (Carassius auratus). Comp. Biochem. Physiol. A. 268, 111189. doi:10.1016/ j.cbpa.2022.111189 Volkoff, H. (2016). The neuroendocrine regulation of food intake in fish: a review of current knowledge. Front. Neurosci. 10, 540. doi:10.3389/fnins.2016.00540 Journal of Experimental Biology (2024) 227, jeb247410. doi:10.1242/jeb.247410 Wang, J., Liang, X.-F., He, S., Zhang, Y.-P., Li, J., Huang, K., Shi, L.-J. and Ren, P. (2020). Valine acts as a nutritional signal in brain to activate TORC1 and attenuate postprandial ammonia-N excretion in Chinese perch (Siniperca chuatsi). Fish. Physiol. Biochem. 46, 2015-2025. doi:10.1007/s10695-02000767-y Wang, Z., Mai, K., Xu, W., Zhang, Y., Liu, Y. and Ai, Q. (2016). Dietary methionine level influences growth and lipid metabolism via GCN2 pathway in cobia (Rachycentron canadum). Aquaculture 454, 148-156. doi:10.1016/j.aquaculture. 2015.12.019 Wei, Y., Sun, Z., Duan, M., Ma, Q., Xu, H. and Liang, M. (2022). Responses to graded levels of leucine and branched-chain amino acid imbalance in tiger puffer Takifugu rubripes. Aquaculture 548, 737699. doi:10.1016/j.aquaculture.2021. 737699 Welcome, M., Mastorakis, O., Pereverzev, N. E. and A, V. (2015). Sweet taste receptor signaling network: Possible implication for cognitive functioning. Neurol. Res. Int. 2015, 606479. doi:10.1155/2015/606479 Williams, I., Williams, K. C., Smith, D. M. and Jones, M. (2006). Polka-dot grouper, Cromileptes altivelis, can utilize dietary fat efficiently. Aquac. Nutr. 12, 379-387. doi:10.1111/j.1365-2095.2006.00437.x Xu, C., Liu, W. B., Zhang, D. D., Wang, K. Z., Xia, S. L. and Li, X. F. (2017). Molecular characterization of AMP-activated protein kinase α2 from herbivorous fish Megalobrama amblycephala and responsiveness to glucose loading and dietary carbohydrate levels. Comp. Biochem. Physiol. A 208, 24-34. doi:10.1016/ j.cbpa.2017.03.008 Xu, H., Sun, B., Liao, Z., Pribytkova, E., Zhang, Q., Wei, Y. and Liang, M. (2019). Possible involvement of PKC/MAPK pathway in the regulation of GnRH by dietary arachidonic acid in the brain of male tongue sole Cynoglossus semilaevis. Aquac. Res. 50, 3528-3539. doi:10.1111/are.14307 Yang, Z., Wong, J., Wang, L., Sun, F. and Yue, G. H. (2023). . pomc knockout increases growth in zebrafish. Aquaculture 574, 739707. doi:10.1016/ j.aquaculture.2023.739707 Yue, S., Li, G., He, S. and Li, T. (2022). The central role of mTORC1 in amino acid sensing. Cancer Res. 82, 2964-2974. doi:10.1158/0008-5472.CAN-21-4403 Zhao, Y., Li, J. Y., Jiang, Q., Zhou, X. Q., Feng, L., Liu, Y., Jiang, W. D., Wu, P., Zhou, J., Zhao, J. et al. (2020). Leucine improved growth performance, muscle growth, and muscle protein deposition through AKT/TOR and AKT/FOXO3a signaling pathways in hybrid catfish Pelteobagrus vachelli×Leiocassis longirostris. Cells 9, 327. doi:10.3390/cells9020327 Zou, J.-M., Zhu, Q.-S., Liang, H., Lu, H.-L., Liang, X.-F. and He, S. (2022). Lysine deprivation regulates npy expression via GCN2 signaling pathway in mandarin fish (Siniperca chuatsi). Int. J. Mol. Sci. 23, 6727. doi:10.3390/ijms23126727 Journal of Experimental Biology REVIEW 17

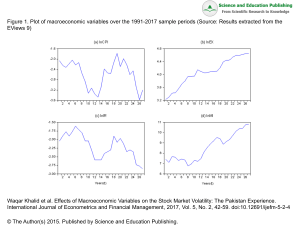

![[PDF Download] Rainbow Milk BY : Paul Mendez](http://s1.studylibid.com/store/data/004534371_1-88f50a1ba748ca5fd7d3fcaefc6b1bd0-300x300.png)