Pemodelan Pengaruh Ikatan H terhadap Waktu Relaksasi T1 13C
advertisement
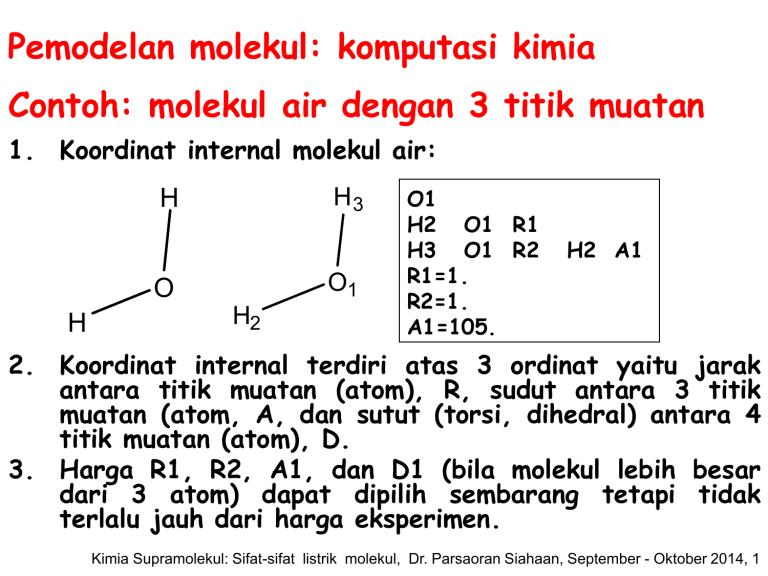
Pemodelan molekul: komputasi kimia Contoh: molekul air dengan 3 titik muatan 1. Koordinat internal molekul air: H3 H O1 O H H2 O1 H2 O1 R1 H3 O1 R2 R1=1. R2=1. A1=105. H2 A1 2. Koordinat internal terdiri atas 3 ordinat yaitu jarak antara titik muatan (atom), R, sudut antara 3 titik muatan (atom, A, dan sutut (torsi, dihedral) antara 4 titik muatan (atom), D. 3. Harga R1, R2, A1, dan D1 (bila molekul lebih besar dari 3 atom) dapat dipilih sembarang tetapi tidak terlalu jauh dari harga eksperimen. Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 1 Pemodelan molekul: komputasi kimia 4. Perhitungan mekanika kuantum ab initio: Input file: misalnya air_opt.g03 #T RHF/6-31G(D,P) opt air opt 0 1 o1 h2 o1 1. h3 o1 1. h2 105. Perintah perhitungan (harus) Tidak harus ditulis Muatan dan multiplisitas (harus) Koordinat internal (harus) Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 2 Pemodelan molekul: komputasi kimia 5. Perhitungan mekanika kuantum ab initio: Output file: misalnya air_opt.out Bagian awal output: #T RHF/6-31G(D,P) opt air opt Symbolic Z-matrix: Charge = 0 Multiplicity = 1 o1 h2 o1 1. h3 o1 1. h2 105. Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 3 Pemodelan molekul: komputasi kimia 5. Perhitungan mekanika kuantum ab initio: Output file: misalnya air_opt.out Bagian output (lanjut): Perhitungan awal: koordinat internal diubah menjadi koordinat kartesian pertama. awal: Standard orientation: ------------------------------------------------------Center Atomic Atomic Coordinates (Angstroms) Number Number Type X Y Z ------------------------------------------------------1 8 0 0.000000 0.000000 0.121752 2 1 0 0.000000 0.793353 -0.487009 3 1 0 0.000000 -0.793353 -0.487009 ------------------------------------------------------Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 4 Pemodelan molekul: komputasi kimia 5. Perhitungan mekanika kuantum ab initio: Bagian output (lanjut): perhitungan awal: konstanta rotasi hingga momen dipol. Rotational constants (GHZ): 761.8214656 398.3536020 261.5763201 SCF Done: E(RHF)=-76.0172883749 A.U. after 10 cycles Convg= 0.4337D-08 -V/T = 2.0048 S**2 = 0.0000 Mulliken atomic charges: 1 O -0.687056 2 H 0.343528 3 H 0.343528 Dipole moment (field-independent basis, Debye): X= 0.0000 Y= 0.0000 Z= -2.2089 Tot= 2.2089 Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 5 Pemodelan molekul: komputasi kimia 5. Perhitungan mekanika kuantum ab initio: Bagian output (lanjut): Step perhitungan 1: konvergensi. Step number 1 out of a maximum of 20 Variable Old X -DE/DX Delta X Delta X (Linear) (Quad) R1 1.88973 -0.05532 0.00000 -0.11303 R2 1.88973 -0.05532 0.00000 -0.11303 A1 1.83260 -0.00316 0.00000 -0.01833 Item Value Threshold Maximum Force 0.055321 0.000450 RMS Force 0.045207 0.000300 Maximum Displacement 0.090403 0.001800 RMS Displacement 0.085525 0.001200 Delta X (Total) -0.11303 -0.11303 -0.01833 Converged? NO NO NO NO New X 1.77670 1.77670 1.81426 Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 6 Pemodelan molekul: komputasi kimia 5. Perhitungan mekanika kuantum ab initio: Bagian output (lanjut): Step perhitungan 1: koordinat kartesian baru (kedua). Standard orientation: -----------------------------------------------------Center Atomic Atomic Coordinates (Angstroms) Number Number Type X Y Z ------------------------------------------------------1 8 0 0.000000 0.000000 0.115833 2 1 0 0.000000 0.740624 -0.463330 3 1 0 0.000000 -0.740624 -0.463330 ------------------------------------------------------Rotational constants (GHZ): 841.6775412 457.0952421 296.2233305 Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 7 Pemodelan molekul: komputasi kimia 5. Perhitungan mekanika kuantum ab initio: Bagian output (lanjut): Step perhitungan 1: Energi. SCF Done: E(RHF) =-76.0234723584 A.U. after 10 cycles Convg=0.3593D-08 -V/T = 2.0019 S**2 = 0.0000 Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 8 Pemodelan molekul: komputasi kimia 5. Perhitungan mekanika kuantum ab initio: Bagian output (lanjut): Step perhitungan 2: konvergensi. Step number 2 out of a maximum of 20 Variable Old X -DE/DX Delta X (Linear) R1 1.77670 0.00455 0.00791 R2 1.77670 0.00455 0.00791 A1 1.81426 0.00674 0.00128 Item Value Maximum Force 0.006742 RMS Force 0.005383 Maximum Displacement 0.024153 RMS Displacement 0.022831 Delta X Delta X (Quad) (Total) -0.00302 0.00489 -0.00302 0.00489 0.03657 0.03785 Threshold Converged? 0.000450 NO 0.000300 NO 0.001800 NO 0.001200 NO New X 1.78159 1.78159 1.85212 Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 9 Pemodelan molekul: komputasi kimia 5. Perhitungan mekanika kuantum ab initio: Bagian output (lanjut): Step perhitungan 2: koordinat kartesian baru (ketiga). Standard orientation: -------------------------------------------------------Center Atomic Atomic Coordinates (Angstroms) Number Number Type X Y Z -------------------------------------------------------1 8 0 0.000000 0.000000 0.113319 2 1 0 0.000000 0.753520 -0.453278 3 1 0 0.000000 -0.753520 -0.453278 -------------------------------------------------------Rotational constants (GHZ): 879.4252319 441.5831829 293.9719298 Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 10 Pemodelan molekul: komputasi kimia 5. Perhitungan mekanika kuantum ab initio: Bagian output (lanjut): Step perhitungan 2: Energi. SCF Done: E(RHF) =-76.0236143166 A.U. after 8 cycles Convg=0.6467D-08 -V/T = 2.0021 S**2 = 0.0000 Bandingkan dengan perhitungan awal dan step 1: SCF Done: E(RHF)=-76.0172883749 A.U. after 10 cycles Convg= 0.4337D-08 -V/T = 2.0048 S**2 = 0.0000 SCF Done: E(RHF) =-76.0234723584 A.U. after 10 cycles Convg=0.3593D-08 -V/T = 2.0019 S**2 = 0.0000 Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 11 Pemodelan molekul: komputasi kimia 5. Perhitungan mekanika kuantum ab initio: Bagian output (lanjut): Step perhitungan 3: konvergensi. Step number 3 out of a maximum of 20 Variable Old X -DE/DX Delta X Delta X Delta X (Linear) (Quad) (Total) R1 1.78159 0.00026 -0.00022 0.00091 0.00069 R2 1.78159 0.00026 -0.00022 0.00091 0.00069 A1 1.85212 -0.00043 -0.00171 -0.00109 -0.00280 Item Value Threshold Converged? Maximum Force 0.000434 0.000450 YES RMS Force 0.000326 0.000300 NO Maximum Displacement 0.001271 0.001800 YES RMS Displacement 0.001373 0.001200 NO New X 1.78227 1.78227 1.84932 Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 12 Pemodelan molekul: komputasi kimia 5. Perhitungan mekanika kuantum ab initio: Bagian output (lanjut): Step perhitungan 3: koordinat kartesian baru (ketiga). Standard orientation: -------------------------------------------------------Center Atomic Atomic Coordinates (Angstroms) Number Number Type X Y Z -------------------------------------------------------1 8 0 0.000000 0.000000 0.113574 2 1 0 0.000000 0.753017 -0.454295 3 1 0 0.000000 -0.753017 -0.454295 -------------------------------------------------------Rotational constants (GHZ): 875.4913660 442.1729232 293.7915065 Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 13 Pemodelan molekul: komputasi kimia 5. Perhitungan mekanika kuantum ab initio: Bagian output (lanjut): Step perhitungan 3: Energi. SCF Done: E(RHF) =-76.0236150032 A.U. after Convg =0.5596D-08 -V/T = 2.0021 S**2 7 cycles = 0.0000 Bandingkan dengan perhitungan awal, step 1, dan step 2: SCF Done: E(RHF)=-76.0172883749 A.U. after 10 cycles Convg= 0.4337D-08 -V/T = 2.0048 S**2 = 0.0000 SCF Done: E(RHF) =-76.0234723584 A.U. after 10 cycles Convg=0.3593D-08 -V/T = 2.0019 S**2 = 0.0000 SCF Done: E(RHF) =-76.0236143166 A.U. after 8 cycles Convg=0.6467D-08 -V/T = 2.0021 S**2 = 0.0000 Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 14 Pemodelan molekul: komputasi kimia 5. Perhitungan mekanika kuantum ab initio: Bagian output (lanjut): Step perhitungan 4: konvergensi. Step number 4 out of a maximum of 20 Variable Old X -DE/DX Delta X Delta X Delta X (Linear) (Quad) (Total) R1 1.78227 -0.00009 -0.00007 -0.00008 -0.00016 R2 1.78227 -0.00009 -0.00007 -0.00008 -0.00016 A1 1.84932 0.00002 0.00030 -0.00013 0.00018 Item Value Threshold Converged? Maximum Force 0.000092 0.000450 YES RMS Force 0.000076 0.000300 YES Maximum Displacement 0.000117 0.001800 YES RMS Displacement 0.000107 0.001200 YES Optimization completed. New X 1.78212 1.78212 1.84950 Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 15 Pemodelan molekul: komputasi kimia 5. Perhitungan mekanika kuantum ab initio: Bagian output (lanjut): Step perhitungan 4: parameter optimasi. -- Stationary point found. ---------------------------! Optimized Parameters ! ! (Angstroms and Degrees) ! ----------------------------------! Name Definition Value Derivative Info. ! ------------------------------------------------------! R1 R(1,2) 0.9431 -DE/DX = -0.0001 ! ! R2 R(1,3) 0.9431 -DE/DX = -0.0001 ! ! A1 A(2,1,3) 105.9583 -DE/DX = 0.0 ! -------------------------------------------------------Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 16 Pemodelan molekul: komputasi kimia 5. Perhitungan mekanika kuantum ab initio: Bagian output (lanjut): Step perhitungan 4: koordinat kartesian baru (keempat). 1 2 3 O H H Distance matrix (angstroms): 1 2 3 0.000000 0.943138 0.000000 0.943138 1.506035 0.000000 Framework group C2V[C2(O),SGV(H2)] Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 17 Pemodelan molekul: komputasi kimia 5. Perhitungan mekanika kuantum ab initio: Bagian output (lanjut): Step perhitungan 4: koordinat kartesian baru (keempat). Standard orientation: -------------------------------------------------------Center Atomic Atomic Coordinates (Angstroms) Number Number Type X Y Z -------------------------------------------------------1 8 0 0.000000 0.000000 0.113574 2 1 0 0.000000 0.753017 -0.454295 3 1 0 0.000000 -0.753017 -0.454295 -------------------------------------------------------Rotational constants (GHZ): 875.4913660 442.1729232 293.7915065 Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 18 Pemodelan molekul: komputasi kimia 5. Perhitungan mekanika kuantum ab initio: Bagian output (lanjut): Step perhitungan 4: analisis populasi. **************************************************************** Population analysis using the SCF density. **************************************************************** Orbital symmetries: Occupied (A1) (A1) (B2) (A1) (B1) Virtual (A1) (B2) (B2) (A1) (A1) (B1) (B2) (A1) (A2) (A1) (B1) (B2) (A1) (B2) (B1) (A2) (A1) (A1) (B2) (A1) The electronic state is 1-A1. Alpha occ. eigenvalues -- -20.55729 -1.34643 -0.71383 -0.56829 -0.49760 Alpha virt. eigenvalues -0.21531 0.30842 1.01682 1.09298 1.13457 Alpha virt. eigenvalues -1.16898 1.29552 1.41170 1.80255 1.82943 Alpha virt. eigenvalues -1.93143 2.58264 2.58874 2.84147 2.99751 Alpha virt. eigenvalues -3.00620 3.40640 3.74549 3.94511 4.12833 Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 19 Pemodelan molekul: komputasi kimia 5. Perhitungan mekanika kuantum ab initio: Bagian output (lanjut): Step perhitungan 4: muatan atom Mullikan dan momen dipol. **************************************************************** Population analysis using the SCF density. **************************************************************** Mulliken atomic charges: (step awal) 1 O -0.670704 2 H 0.335352 3 H 0.335352 Mulliken atomic charges: (step 4) 1 O -0.687056 2 H 0.343528 3 H 0.343528 Dipole moment (field-independent basis, Debye): X= 0.0000 Y= 0.0000 Z= -2.1478 Tot= 2.1478 Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 20 Pemodelan molekul: komputasi kimia 5. Perhitungan mekanika kuantum ab initio: Output file: misalnya air_opt.out AND HERE I AM, FOR ALL MY LORE, THE WRETCHED FOOL I WAS BEFORE. CALLED MASTER OF ARTS, AND DOCTOR TO BOOT, FOR TEN YEARS ALMOST I CONFUTE AND UP AND DOWN, WHEREVER IT GOES I DRAG MY STUDENTS BY THE NOSE -- AND SEE THAT FOR ALL OUR SCIENCE AND ART WE CAN KNOW NOTHING. IT BURNS MY HEART. -- FAUST Job cpu time: 0 days 0 hours 0 minutes 4.2 seconds. File lengths (MBytes): RWF= 11 Int= 0 D2E= 0 Chk= 4 Scr= 1 Normal termination of Gaussian 03 at Wed Apr 8 11:35:24 2009. Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 21 Polarisasi The polarization, P (Cm/m3) of a sample is the electric dipole moment density, the mean electric dipole moment of molecules,<μ>, multiplied by the number density, N (m-3): P N Persamaan 6 Pada temperatur T: z 3kT Persamaan 7 Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 22 Polarisabilitas An applied electric field can distort a molecule as well as align its permanent electric dipole moment. The induced dipole moment, μ* (Cm), is generally proportional to the field strength, E, and we write: Persamaan 8 The constant of proportionality is the polarizability of the molecule. The greater the polarizability, the larger is the induced dipole moment for a given applied field. In a formal treatment, we should use vector quantities and allow for the possibility that the induced dipole moment might not lie parallel to the applied field, but for simplicity we discuss polarizabilities in terms of (scalar) magnitudes. Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 23 Polarisabilitas Volume Polarizability has the units (coulomb metre)2 per joule (C2m2J-1). That collection of units is awkward, so α is often expressed as a polarizability volume, α’, by using the relation: 4 o ' Persamaan 9 where εo is the vacuum permittivity. Because the units of are coulomb-squared per joule per metre (C2J-1m-1), it follows that α has the dimensions of volume (hence its name). Polarizability volumes are similar in magnitude to actual molecular volumes (of the order of 10-30 m3, 10-3 nm3, 1 Ao3. Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 24 Momen Dipol Listrik Tabel 3: Polarizability volumes (α’) Molecules CCl4 H2 H2O HCl HI (1,2) α’/(10-30 m3) 10.5 0.819 1.48 2.63 5.45 Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 25 Polarisabilitas Volume Polarizability volumes correlate with the HOMO-LUMO separations in atoms and molecules. The electron distribution can be distorted readily if the LUMO lies close to the HOMO in energy, so the polarizability is then large. If the LUMO lies high above the HOMO, an applied field cannot perturb the electron distribution significantly, and the polarizability is low. Molecules with small HOMO-LUMO gaps are typically large, with numerous electrons. Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 26 Polarisabilitas dan Struktur Molekul The contribution to the Hamiltonian when a dipole moment is exposed to an electric field in the zdirection is (1) Persamaan 11 H z By a series of the mathematical operation, the polarizability of the molecule in the z-direction is 2 n z ,0 n (0) n E 2 E (0) 0 Persamaan 12 where μz,0n is the transition electric dipole moment in the z-direction. Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 27 Polarisabilitas dan Struktur Molekul The content of eqn previous can be appreciated by approximating the exitation energies by a mean value ΔE (an indication of the HOMO-LUMO separation), and supposing that the most important transition dipole moment is approximately equal to the charge of an electron multiplied by the radius, R, of the molecule. Then is 2e2 R 2 E Persamaan 13 This expression shows that α increases with size of the molecule and with the ease with which it can be excited (the smaller the value of ΔE). Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 28 Polarisabilitas dan Struktur Molekul If the excitation energy is approximated by the energy needed to remove an electron to invinity from a distance R from a single positive charge, we can write: 2 Persamaan 14 o E e 4 R When this expression is substituted into the equation previous, and 9, and the factor 2 ignored in this approximation, we obtain, R ' 3 Persamaan 15 which is of the same order of magnitude as the molecular volume). Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 29 Polarisabilitas dan Struktur Molekul For most molecules, the polarizability is anisotropic, by which is meant that its value depends on the orientation of the molecule relative to the field. The polarizability volume of benzene when the field is applied perpendicular to the ring is 0.0067 nm3 and it is 0.0123 nm3 when the field is applied in the plane of the ring. The anisotropy of the polarizability determines whether a molecule is rotationally Raman active. Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 30 Permitivitas Relatif Potential energy of interaction of two charges q1 and q2 which are separated by a distance r in a vacuum is q1q2 V 4 o r Persamaan 16 where εo is the vacuum permittivity. In a medium such as air or a liquid: q1q2 V 4 r Persamaan 17 where ε is the permittivity of the medium. Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 31 Permitivitas Relatif Permitivitas dapat dinyatakan dalam permitivitas relatif (tak berdimensi), εr, (tetapan dielektrik) medium: r o Persamaan 18 The relative permittivity can have a very significant effect on the strength of the interactions between ions in solutions. For instance, water has a relative permittivity of 78 at 25 oC, so the interionic Coulombic interaction energy is reduced by nearly two orders (102) of magnitude from its vacuum value. Some of the consequences of this reduction for electrolyte solutions were explored in chapter of simple mixtures. . Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 32 Permitivitas Relatif The relative permittivity of a substance is large if its molecules are polar or highly polarizable. The quantitative relation between the relative permittivity and the electric properties of the molecules is obtained by considering the polarization of a medium, and is expressed by the Debye equation: r 1 Pm r 2 M Persamaan 19 where ρ is the mass density of the sample, M is the molar mass of the molecules, and Pm is the molar polarization. Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 33 Permitivitas Relatif Pm is the molar polarization, which defined as: 2 NA Pm 3 o 3kT Persamaan 20 The term 2 3kT stems from the termal averaging of the electric dipole moment in the presence of the applied field (eqn 7). The corresponding expression without the contribution from the permanent dipole moment is called the Clausius-Mossotti equation: r 1 N A r 2 3M o Persamaan 21 Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 34 Permitivitas Relatif The Clausius-Mossotti equation is used when there is no contribution from permanent electric dipole moment to the polarization, either because the molecules are non-polar or because the frequency of the applied field is so high that the molecule can not orientate quickly enough to follow the change in direction of the field. Contoh: Permitivitas relatif suatu zat diukur dengan membandingkan kapasitansi kapasitor dengan dan tanpa adanya sampel masingmasing C dan Co menggunakan hubungan: r C Co Permitivitas relatif kamper diukur pada berbagai temperatur, tabel 4. Tentukan momen dipol dan polarisabilitas volume molekul. Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 35 Contoh: T/oC ρ/(gcm-3) 0 20 40 60 80 100 120 140 160 200 0.99 0.99 0.99 0.99 0.99 0.99 0.97 0.96 0.95 0.91 εr=C/Co 12.50 11.40 10.80 10.00 9.50 8.90 8.10 7.60 7.11 6.21 Equation 19 implies that the polarizability and permanent electric dipole moment of the molecules in a sample can be determined by measuring r at a series of temperatures, calculating Pm, and plotting it against 1/T. The slope of the graph is N A 2 9 o k and its intercept at 1/T=0 is N A 3 o We need to calculate r 1 r 2 at each temperature, and then multiply by M/ρ to form Pm. Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 36 Jawab: T/oC 0 20 40 60 80 100 120 140 160 200 (103 K)/T 3.66 3.41 3.19 3.00 2.83 2.68 2.54 2.42 2.31 2.11 εr (εr -1)/(εr +2) 12.50 0.793 11.40 0.776 10.80 0.766 10.00 0.750 9.50 0.739 8.90 0.725 8.10 0.703 7.60 0.688 7.11 0.670 6.21 0.634 Pm/(cm3 mol-1) 122 119 118 115 114 111 110 109 107 106 Hasilnya adalah: 1. momen dipol μ= 4,46.10 -30 2. Polarizabilitas, α’ = 3,3.10 Cm = 1,34 D. -23 3 cm . Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 37 Tugas 1 1. Gunakan software kimia untuk menentukan kepolaran molekul: • ClF3, O3, H2O2. • SO3, XeF4, SF4. • Metil benzena (toluena), dimetil benzena. 2. Polarisasi molar (persamaan 20) uap fluorobenzena berubah secara linier terhadap T-1, yaitu 70,62 cm3/mol pada 351,0K dan 62,47 cm3/mol pada 423,2K. Hitung polarisabilitas dan momen dipol molekul. 3. Pada 0oC, polarisasi molar trifluoroklorida cair adalah 27,18 cm3/mol dan kerapatannya 1,89 g/cm3. Hitung permitivitas relatif cairan. 4. Polarisabilitas volume H2O adalah 1,48.10-24 cm3. Hitung momen dipol molekul (tambahan pada momen dipol permanen) yang diinduksi oleh medan listrik dengan kekuatan 1,0 kV/cm. Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 38 Tugas 2 1. Polarisabilitas volume NH3 adalah 2,22.10-30 cm3. Hitung momen dipol molekul (tambahan pada momen dipol permanen) yang diinduksi oleh medan listrik dengan kekuatan 15,0 kV/cm. 2. Momen dipol dan polarisabilitas volume klorobenzena masing-masing adalah 1,57 D dan 1,23.10-23 cm3. Perkirakan permitivitas relatifnya pada 25 oC, bila kerapatannya 1,173 g/cm3. 3. Momen dipol dan polarisabilitas volume bromobenzena masing-masing adalah 5,17.10-30 Cm dan 1,5.10-29 m3. Perkirakan permitivitas relatifnya pada 25 oC, bila kerapatannya 1491 kg/m3. Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 39 Tugas 3 1. Molekul H2O (μ=1,85 D) mendekati suatu anion. Bagaimana orientasi molekul H2O yang lebih disukai? Hitung medan listrik (dalam volt/m) yang dialami oleh anion bila dipol air berjarak : (a) 1,0 nm, (b) 0,3 nm, dan (c) 30 nm dari ion. 2. Perhitungan orbital molekul dapat digunakan untuk memprediksi struktur kompleks intermolekul. Ikatan-H antara basa purin dan pirimidin adalah penyebab struktur double helix DNA. Anggap bahwa metil-adenin dan metil-timin sebagai model kedua basa yang dapat membentuk ik-H dalam DNA. Gunakan software pemodelan molekul dan metoda komputasi untuk: (a). menghitung muatan atom pada metil-adenin dan metil-timin. (b). Berdasarkan tabulasi muatan atom, tunjukkan atom-atom dalam metil-adenin dan metil-timin yang mungkin terlibat dalam ikatan-H. (c). Gambarkan semua kemungkinan pasangan adenintimin yang dapat diikat ikatan-H, dengan menganggap penataan linier fragmen A-H...B lebih disukai dalam DNA. (d) Lakukan optimasi untuk panataan molekul yang lebih tepat. (e) bandingkan hasil (c) dan (d) dengan DNA yang terjadi secara alami. (e). Lakukan (a)-(e) untuk pasangan sitosin dan guanin. Kimia Supramolekul: Sifat-sifat listrik molekul, Dr. Parsaoran Siahaan, September - Oktober 2014, 40